| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 4, August 2025, pages 214-222
Efficacy and Safety of Momelotinib in Myelofibrosis: A Systematic Review and Meta-Analysis With a Focus on Anemia Outcomes
Jowan Al-Nusaira, d, e, Mo’men Aldalal’ahb, d, Mohammad Alqudahb, Fakhri Al-Malkawib, Nora Al-Khateebb, Hasan Khasawnehb, Omar El-Shatelb, Obada Khayyatb, Sarah H. Alseidb, Mahmoud Abdallahc, Ola Soudahb, Toni Paciolesc, Muhammad Omer Jamilc
aDepartment of Internal Medicine, Marshall University Joan C. Edwards School of Medicine, Huntington, WV 25701, USA
bFaculty of Medicine, Yarmouk University, Irbid, Jordan
cDepartment of Oncology, Edwards Comprehensive Cancer Institute, Marshall University, Huntington, WV 25701, USA
dThese authors contributed equally to this work.
eCorresponding Author: Jowan Al-Nusair, Department of Internal Medicine, Marshall University Joan C. Edwards School of Medicine, Huntington, WV 25701, USA
Manuscript submitted June 25, 2025, accepted July 24, 2025, published online August 25, 2025
Short title: Efficacy and Safety of Momelotinib in MF
doi: https://doi.org/10.14740/jh2094
| Abstract | ▴Top |
Background: Myelofibrosis (MF) can be primary (PMF) or secondary (SMF), with PMF driven by Janus kinases-signal transducer and activator of transcription proteins (JAK-STAT) pathway activation due to Janus kinase 2 (JAK2), the thrombopoietin receptor gene (myeloproliferative leukemia virus oncogene (MPL)), or calreticulin (CALR) mutations. Nearly 50% of PMF patients experience anemia (hemoglobin (Hb) < 10 g/dL), often worsened by JAK inhibitors like ruxolitinib and fedratinib. Momelotinib, an oral ACVR1, JAK1, and JAK2 inhibitor, improves anemia, symptoms, and splenomegaly, likely through hepcidin regulation. This review evaluates its efficacy and safety, with a focus on anemia.
Methods: A systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including randomized controlled trials (RCTs) and clinical studies assessing momelotinib’s efficacy and safety. Primary outcomes included spleen volume reduction (≥ 35%) and anemia response (transfusion independence). Secondary endpoints included symptom burden reduction and safety.
Results: Six studies, including three RCTs, met inclusion criteria. Meta-analysis showed momelotinib was noninferior to ruxolitinib in spleen volume reduction but superior in anemia benefits, increasing transfusion independence (odds ratio (OR): 2.09; 95% confidence interval (CI): 1.53 - 2.85) and reducing transfusion dependence (OR: 0.62; 95% CI: 0.45 - 0.84). Symptom burden reduction was comparable to other JAK inhibitors. Common adverse events included dizziness (OR: 1.70; 95% CI: 1.05 - 2.74) and nausea (OR: 3.07; 95% CI: 1.82 - 5.18), with no significant increase in serious adverse events.
Conclusions: Momelotinib improved anemia-related outcomes and quality of life in MF without increased adverse events. However, heterogeneity in control groups limited direct efficacy comparisons. Larger studies are needed to confirm its effectiveness and safety.
Keywords: Myelofibrosis; Momelotinib; Anemia; Myeloproliferative neoplasms
| Introduction | ▴Top |
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm (MPN) characterized by progressive bone marrow fibrosis (BMF), cytopenias, extramedullary hematopoiesis, and debilitating constitutional symptoms [1]. The disease is driven by dysregulated signaling by Janus kinases-signal transducer and activator of transcription proteins (JAK-STAT), commonly resulting from mutations in Janus kinase 2 (JAK2), calreticulin (CALR), or thrombopoietin receptor gene (myeloproliferative leukemia virus oncogene (MPL)), leading to ineffective hematopoiesis and chronic inflammation [2]. The clinical course of MF is variable; some patients experience stable disease, while others develop severe anemia, thrombocytopenia, or progression to acute myeloid leukemia (AML), which is associated with a poor prognosis [3]. Current treatments mainly aim to relieve symptoms and reduce spleen size, while disease-modifying approaches remain under investigation [4]. Ruxolitinib, a JAK1/2 inhibitor, has been the foundation of MF treatment, demonstrating significant improvements in symptom burden and spleen volume reduction. However, its use is often limited by hematologic toxicities, particularly the worsening of anemia and thrombocytopenia, which may require dose reductions or treatment discontinuation [5]. Other JAK inhibitors, such as fedratinib and pacritinib, have expanded treatment options, but each presents specific safety challenges. Given these limitations, there remains an unmet need for therapies that provide symptom control while improving hematologic parameters, particularly anemia [6]. Momelotinib, a selective JAK1/2 and activin A receptor type 1 (ACVR1) inhibitor, has emerged as a promising therapy for MF by targeting anemia through hepcidin suppression and enhanced erythropoiesis [7]. Clinical trials have shown that momelotinib offers comparable spleen volume reduction to ruxolitinib while providing superior benefits for anemia, positioning it as a valuable option for patients with transfusion dependence [8]. Despite these advantages, uncertainties remain regarding its overall efficacy in symptom improvement and potential for long-term disease modification. This systematic review and meta-analysis aim to comprehensively evaluate the efficacy and safety of momelotinib in MF, with a particular focus on spleen volume reduction, symptom burden, and anemia-related outcomes. By synthesizing data from randomized controlled trials (RCTs) and clinical studies, this analysis seeks to clarify momelotinib’s clinical role, highlight its strengths and limitations, and inform future directions for MF management.
| Materials and Methods | ▴Top |
Institutional Review Board approval was not required for this study, as it is based entirely on publicly available data from previously published studies. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9] and the Cochrane Handbook of Systematic Reviews and Meta-Analyses [10]. To minimize bias and ensure transparency, the study was registered with the International Registration of Systematic Reviews (PROSPERO) under ID CRD4202459139.
A comprehensive search was conducted in PubMed, Scopus, the Cochrane Central Register of Controlled Trials (CENTRAL), and the US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov), covering all available records up to July 2023. The search strategy included key concepts such as “myelofibrosis”, “thrombocytopenia”, “polycythemia vera”, “momelotinib”, and “CYT387”, with a combination of free-text terms, truncated words, and medical subject heading (MeSH) terms. Boolean logic operators (AND, OR, NOT) were used to refine the results, and a customized search strategy was developed for each database (Supplementary Material 1, jh.elmerpub.com).
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
Included studies were RCTs, open-label clinical trials, or non-randomized clinical trials that enrolled adults (≥ 18 years) diagnosed with primary MF, post-essential thrombocythemia MF, or post-polycythemia vera MF, according to World Health Organization (WHO) criteria [11]. The intervention was momelotinib administered at any dose, with comparator groups including other available therapies, placebo, or no treatment.
The primary outcome was a ≥ 35% reduction in spleen volume from baseline at week 24. Secondary outcomes included the proportion of patients achieving a ≥ 50% reduction in total symptom score (TSS) based on the modified Myeloproliferative Neoplasm Symptom Assessment Form TSS diary [12, 13], transfusion independence and dependence rates, and overall response rate per the International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) criteria [14]. Safety was assessed based on the incidence of adverse events (AEs) [15]. Observational studies (cohort, case-control, and cross-sectional) and descriptive reports (case reports and case series) were excluded.
Search results were imported into EndNote, and duplicates were removed. Two independent reviewers screened the titles and abstracts, excluding non-eligible studies. Disagreements during full-text screening and risk of bias assessment were resolved through discussion and consensus among the reviewers, with input from a third reviewer when needed. Full-text screening was subsequently conducted by two additional reviewers, with final eligibility decisions adjudicated when needed. Reference lists of included studies were manually searched for additional relevant articles.
Two independent reviewers performed data extraction using a pre-specified form. Extracted data included study characteristics (country, study design, total participants, inclusion criteria, primary outcome, and follow-up duration), baseline patient characteristics (age, sex, body mass index (BMI), race, ethnicity, MF subtype, IPSS risk category, JAK2V617F mutation status, TSS, Eastern Cooperative Oncology Group (ECOG) performance status, mean hemoglobin, platelet count, and neutrophil count), efficacy outcomes (splenic response, TSS response, transfusion dependence and independence rates), and safety outcomes (AEs and serious adverse effects). In multi-arm studies, data were extracted separately and aggregated to obtain a single outcome measure.
Two reviewers independently assessed study quality using the Cochrane risk-of-bias tool (version 2) [16] for RCTs and open-label trials, evaluating five domains: bias from randomization, deviations from intended interventions, missing outcome data, outcome measurement, and selective reporting. For non-randomized trials, the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool [17] was used to assess seven domains, including confounding, participant selection, intervention classification, deviations from intended interventions, missing data, outcome measurement, and selective reporting. Risk of bias for each domain was classified as low, unclear, or high.
Meta-analysis was performed using R software (version 4.3.1). Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated, with statistical significance set at P < 0.05. Heterogeneity was assessed using the I2 statistic, with thresholds of < 25% indicating low heterogeneity, 25-75% indicating moderate heterogeneity, and > 75% indicating high heterogeneity [10]. A random-effects model was used when heterogeneity was significant, and subgroup analyses were conducted when feasible [18]. In the absence of significant heterogeneity, a fixed-effects model was applied [19]. Sensitivity analyses were performed to identify heterogeneity sources.
| Results | ▴Top |
Search results and study selection
The database search retrieved 433 records. After removing 44 duplicates, 389 records remained for screening. Following title and abstract review, 36 articles were selected for full-text evaluation, and six studies met the inclusion criteria. These included three double-arm RCTs and three single-arm clinical trials [20-26] (Fig. 1). Risk of bias assessments for RCTs and single-arm trials are detailed here (Supplementary Materials 2, 3, jh.elmerpub.com).
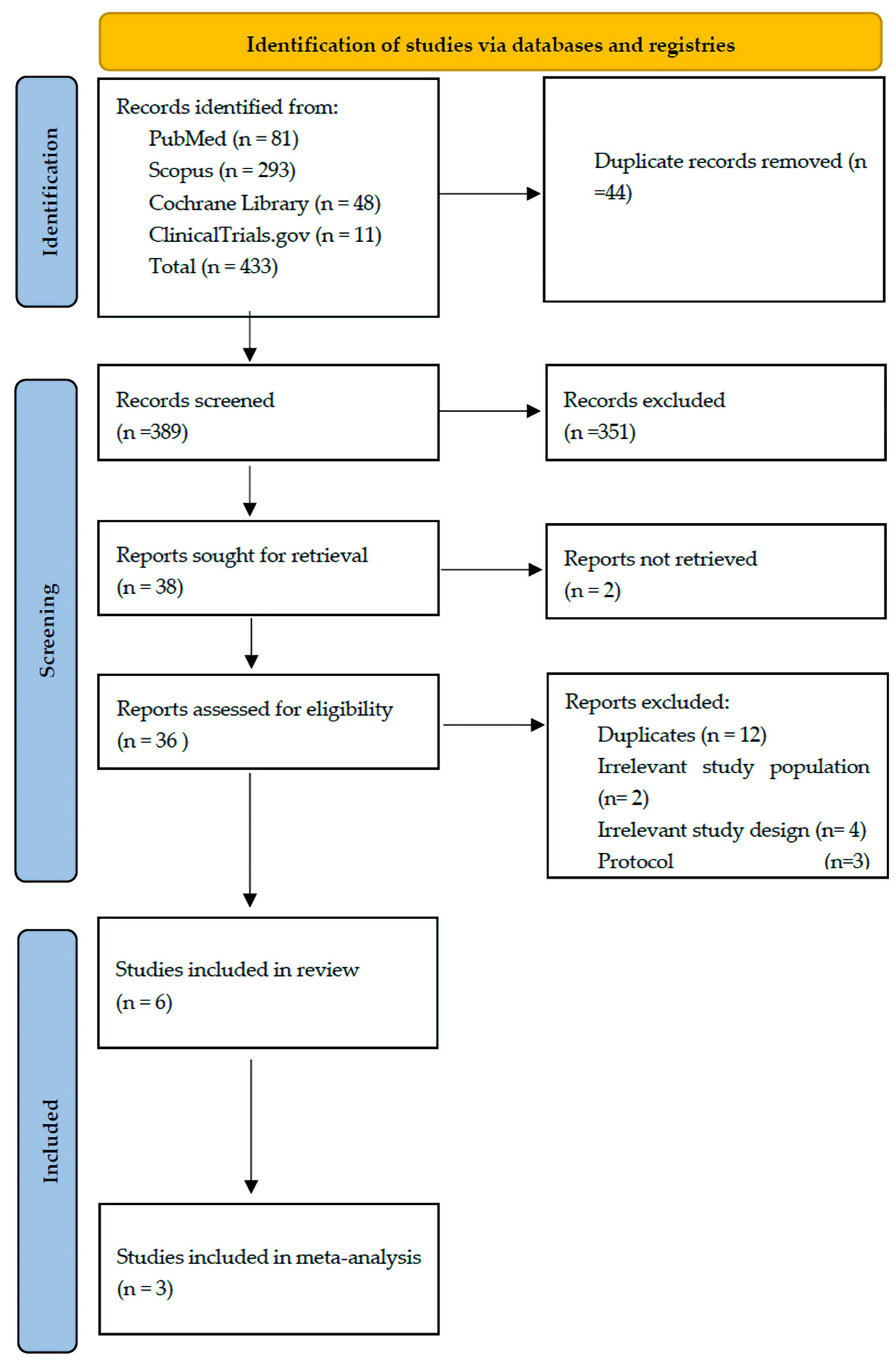 Click for large image | Figure 1. PRISMA chart of the reported studies showing the search selection strategy and exclusion criteria. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Characteristics of included studies
Six studies investigated the efficacy and safety of momelotinib, with three RCTs [21-23] comparing momelotinib to a control group (included in the meta-analysis) and three single-arm studies [20, 24-26] reported in a qualitative synthesis (Supplementary Material 4, jh.elmerpub.com). Baseline characteristics of participants across included trials are presented here (Supplementary Material 5, jh.elmerpub.com), while details the outcomes characteristics for each study are detailed here (Supplementary Material 6, jh.elmerpub.com).
Efficacy outcomes
Momelotinib did not significantly improve the overall response rate compared to the control group (OR: 1.80; 95% CI: 0.72 - 4.46) (Supplementary Material 7, jh.elmerpub.com). The splenic response rate was also not significantly different (OR: 1.96; 95% CI: 0.53 - 7.25) (Fig. 2). Given the high heterogeneity (I2 = 75%), a random-effects model was applied, and sensitivity analysis identified NCT04173494 as a major source of heterogeneity (Supplementary Material 8a, jh.elmerpub.com). However, momelotinib significantly increased transfusion independence (OR: 2.09; 95% CI: 1.53 - 2.85) (Fig. 3) and reduced transfusion dependence (OR: 0.62; 95% CI: 0.45 - 0.84) (Fig. 4). No significant effect was observed for TSS response rate (OR: 2.02; 95% CI: 0.51 - 8.00) (Fig. 5), with high heterogeneity (I2 = 90%) attributed to NCT01969838 (Supplementary Material 8b, jh.elmerpub.com). Due to the limited number of studies, subgroup analyses were not conducted.
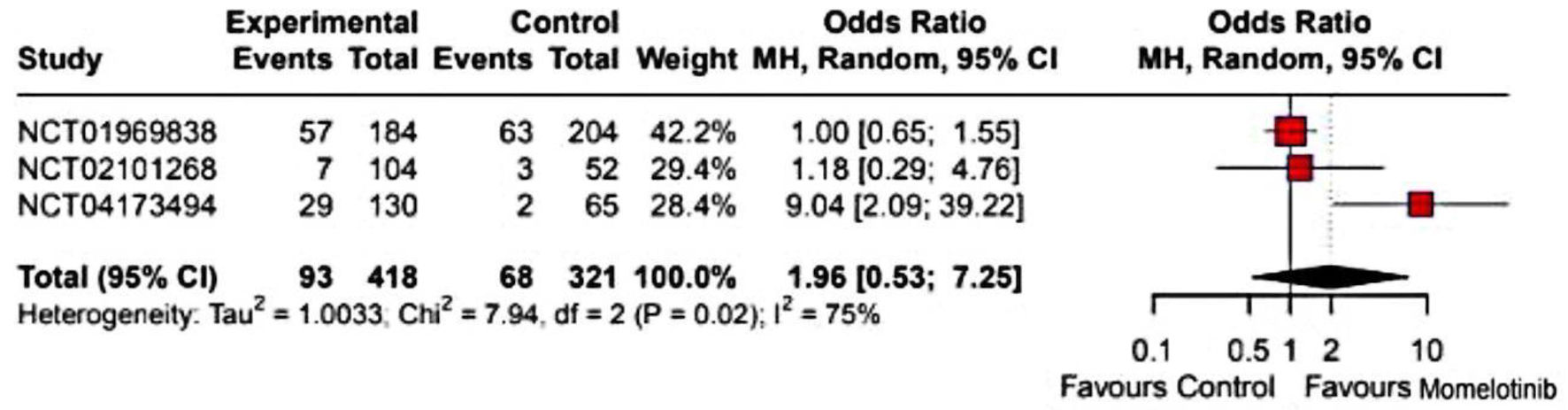 Click for large image | Figure 2. Forest plot of splenic response rate. NCT identifiers correspond to: NCT02101268 - SIMPLIFY-2; NCT01969838 - SIMPLIFY-1; NCT04173494 - MOMENTUM. CI: confidence interval. |
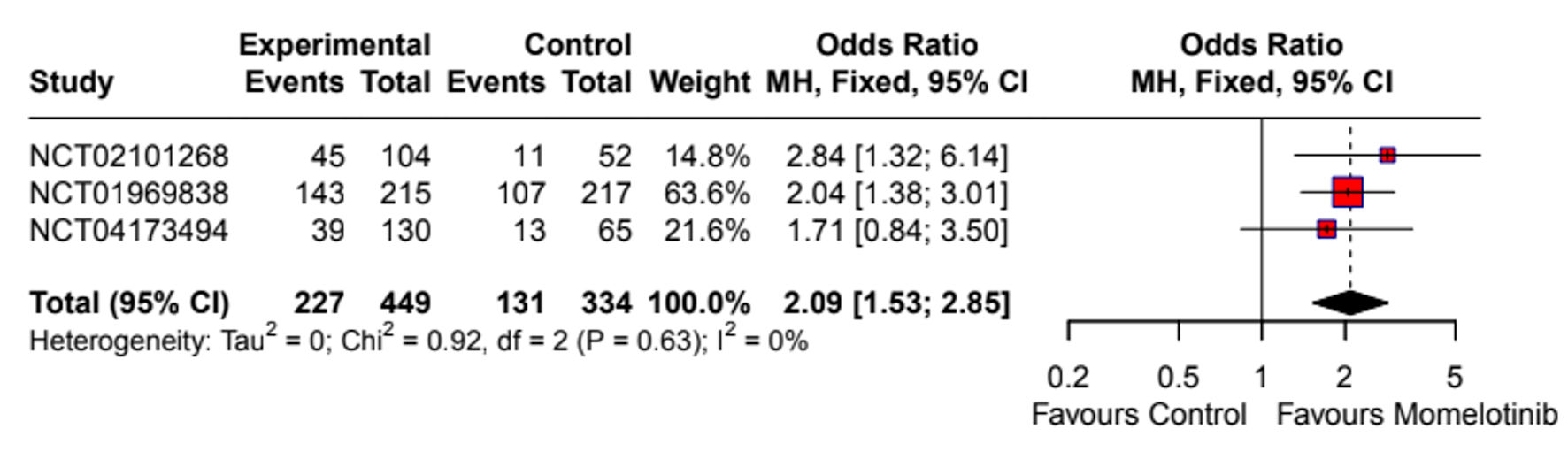 Click for large image | Figure 3. Forest plot of transfusion independence. NCT identifiers correspond to: NCT02101268 - SIMPLIFY-2; NCT01969838 - SIMPLIFY-1; NCT04173494 - MOMENTUM. CI: confidence interval. |
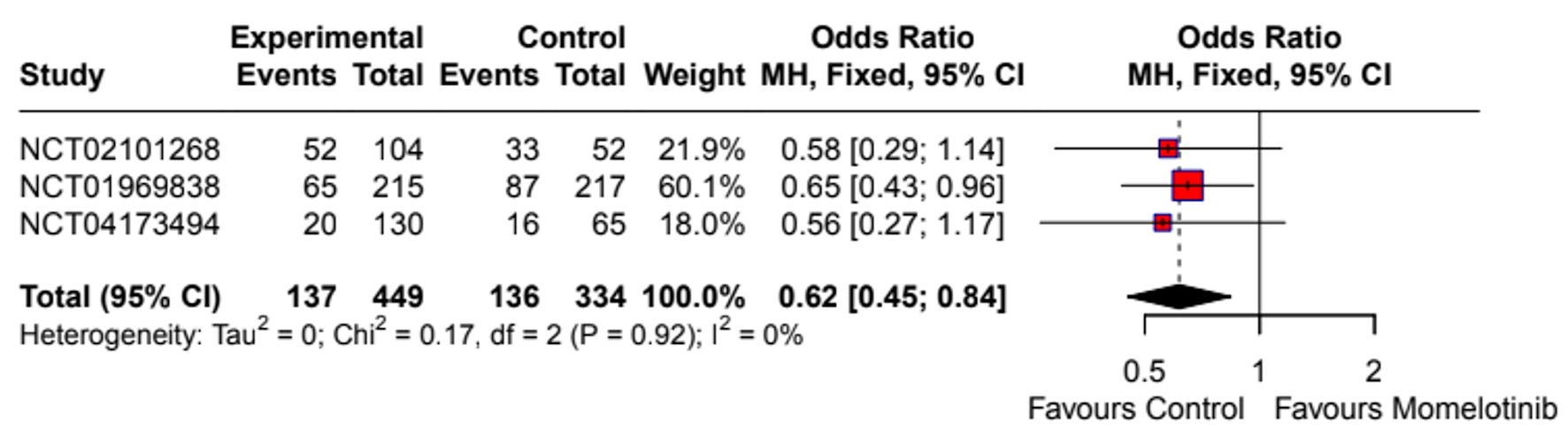 Click for large image | Figure 4. Forest plot of transfusion dependence. NCT identifiers correspond to: NCT02101268 - SIMPLIFY-2; NCT01969838 - SIMPLIFY-1; NCT04173494 - MOMENTUM. CI: confidence interval. |
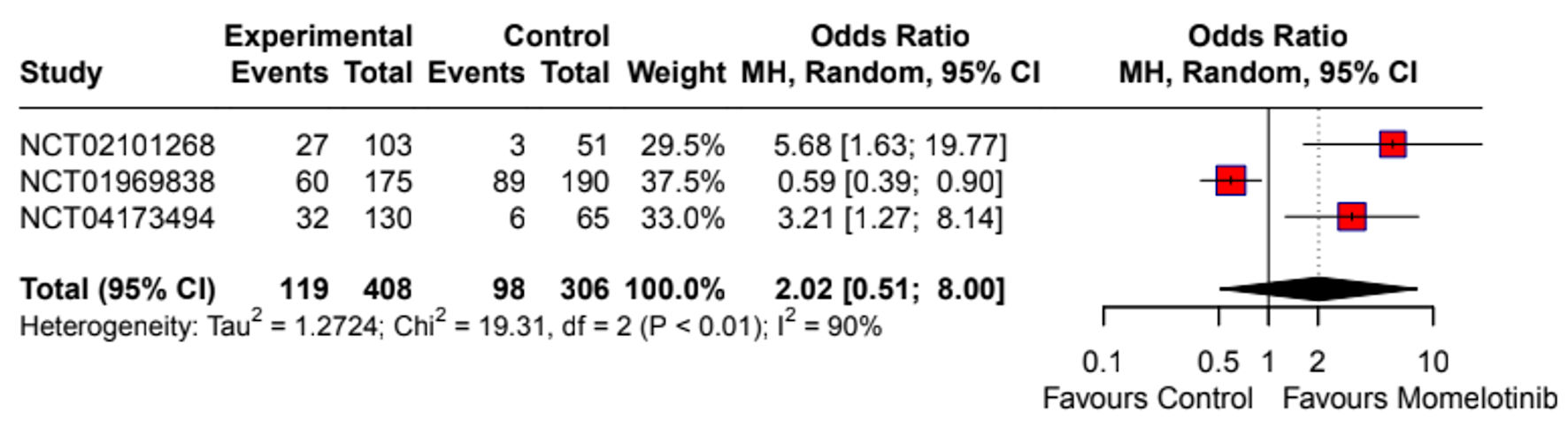 Click for large image | Figure 5. Forest plot of TSS response rate. NCT identifiers correspond to: NCT02101268 - SIMPLIFY-2; NCT01969838 - SIMPLIFY-1; NCT04173494 - MOMENTUM. CI: confidence interval; TSS: total symptom score. |
Safety outcomes
Momelotinib did not significantly alter the overall incidence of AEs (OR: 1.13; 95% CI: 0.39 - 3.30) (Supplementary Material 9a, jh.elmerpub.com). However, dizziness (OR: 1.70; 95% CI: 1.05 - 2.74) (Supplementary Material 9b, jh.elmerpub.com) and nausea (OR: 3.07; 95% CI: 1.82 - 5.18) (Supplementary Material 9c, jh.elmerpub.com) were more common in the momelotinib group. There were no significant differences in drug discontinuation (OR: 2.34; 95% CI: 0.53 - 10.38) (Supplementary Material 9d, jh.elmerpub.com), mortality (OR: 0.73; 95% CI: 0.43 - 1.23) (Supplementary Material 9e, jh.elmerpub.com), dose reduction (OR: 0.89; 95% CI: 0.23 - 3.46) (Supplementary Material 10a, jh.elmerpub.com), or serious AEs (OR: 1.21; 95% CI: 0.81 - 1.82) (Supplementary Material 10b, jh.elmerpub.com). High heterogeneity was observed in several safety outcomes, with sensitivity analyses identifying specific studies as contributors (Supplementary Material 11a-f, jh.elmerpub.com).
| Discussion | ▴Top |
This systematic review and meta-analysis evaluated the efficacy and safety of momelotinib in the treatment of MF, with a focus on its effects on anemia, splenic response, and symptom burden. MF is a chronic MPN characterized by progressive BMF, cytopenias, and constitutional symptoms [27, 28]. While ruxolitinib has been the standard JAK inhibitor for symptomatic MF [29], its limited impact on anemia has led to the exploration of alternative therapies such as momelotinib, a JAK1/2 and ACVR1 inhibitor [30]. Our findings highlight momelotinib’s distinct clinical profile, particularly its benefits for anemia, while identifying areas where its efficacy is comparable or inferior to existing therapies.
Ruxolitinib, the first approved JAK inhibitor, targets JAK1, JAK2, and tyrosine kinase 2 (TYK2) [31]. Pivotal clinical trials, including COMFORT-I and COMFORT-II, led to its Food and Drug Administration (FDA) approval in 2011 for intermediate- and high-risk MF [29]. However, its use is often limited by AEs such as anemia, thrombocytopenia, non-melanoma skin cancers, infections, and hyperlipidemia [32]. Fedratinib, which targets JAK2, JAK3, and FMS-like tyrosine kinase 3 (FLT3), was approved in 2019 based on the JAKARTA-2 and FREEDOM trials and is associated with gastrointestinal toxicity, thiamine deficiency, and cytopenias [33]. Pacritinib, targeting JAK2, interleukin-1 receptor-associated kinase 1 (IRAK1), and ACVR1, was approved in 2022 for MF patients with platelet counts below 50 × 109/L, based on the PERSIST-1, PERSIST-2, and PAC-203 trials, but carries risks of gastrointestinal side effects, cardiac events, QT prolongation, and hemorrhage [34]. Momelotinib, distinct in its ability to address anemia, was approved in 2023 for intermediate- and high-risk MF with anemia based on the SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM trials, with key AEs including gastrointestinal disturbances and cytopenias [35].
A major advantage of momelotinib demonstrated in this analysis is its impact on transfusion independence. Our meta-analysis showed a significant increase in transfusion independence (OR: 2.09; 95% CI: 1.53 - 2.85) and a reduction in transfusion dependence (OR: 0.62; 95% CI: 0.45 - 0.84), emphasizing its role in addressing anemia, a critical issue for MF patients. These results align with the MOMENTUM trial, which demonstrated that momelotinib significantly improved anemia-related outcomes compared to danazol [23]. Additionally, SIMPLIFY-1 demonstrated noninferiority to ruxolitinib in spleen volume reduction, suggesting that momelotinib provides comparable control of splenomegaly while offering additional hematologic benefits [22]. A post-hoc analysis of SIMPLIFY-1 showed that momelotinib was beneficial for JAK inhibitor-naive MF patients regardless of baseline hemoglobin, with greater anemia-related advantages observed in patients with hemoglobin < 12 g/dL [36]. A subgroup analysis of SIMPLIFY-2 similarly found that switching to momelotinib led to improved outcomes compared to continuing ruxolitinib with supportive treatments in patients with moderate-to-severe anemia [37]. However, Oh et al reported that momelotinib’s anemia benefit is independent of changes in BMF, challenging the use of BMF improvement as a surrogate marker for clinical outcomes with JAK inhibitors [38]. Our findings are further supported by real-world data from a recent study by Harrison et al, which assessed transfusion burden over time in both JAK inhibitor-naive and -experienced patients treated with momelotinib [39]. Across the SIMPLIFY-1, SIMPLIFY-2, and MOMENTUM trials, momelotinib consistently reduced or stabilized transfusion requirements in a higher proportion of patients compared to ruxolitinib, best available therapy, and danazol. These time-dependent analyses provide additional evidence of momelotinib’s anemia benefit beyond binomial endpoints.
Despite these advantages, momelotinib did not demonstrate clear superiority over comparator treatments in terms of spleen response or symptom burden. Our meta-analysis found no statistically significant improvement in spleen response (OR: 1.96; 95% CI: 0.53 - 7.25) or TSS reduction (OR: 2.02; 95% CI: 0.51 - 8.00). These results are consistent with SIMPLIFY-1 and SIMPLIFY-2, which reported that momelotinib was comparable to ruxolitinib in spleen response but inferior in symptom improvement [21, 22]. Similarly, the MOMENTUM trial showed that while momelotinib improved symptoms compared to danazol, it did not meet noninferiority criteria when compared to ruxolitinib [23]. Notably, the OR for drug-related mortality (OR 0.73; 95% CI: 0.43 - 1.23), though not statistically significant, slightly favored the comparator. While not conclusive, this finding underscores the need for continued safety monitoring and further comparative studies.
Combination therapy strategies have been increasingly explored in MF management following the approval of ruxolitinib [40]. Approaches such as combining ruxolitinib with erythropoiesis-stimulating agents (ESAs) [41], danazol, or immunomodulatory drugs (IMiDs) for anemia [40], and ruxolitinib combined with hypomethylating agents such as azacitidine [42] or decitabine [43] for accelerated or blast-phase MF, are under investigation. Ongoing phase 3 trials are evaluating JAK inhibitor combinations with novel agents aimed at disease modification and overall survival improvement, including immune-directed therapies such as interferon (IFN) formulations [44] and CALR-targeted vaccines [45]. Additionally, novel therapeutic targets and mutant-specific platforms are being developed [46]. Future treatment paradigms are expected to focus on broader endpoints beyond spleen and symptom control, including overall survival, progression-free survival, event-free survival, AML transformation rates, anemia improvement, transfusion burden reduction, driver variant allele frequency (VAF) reduction, cytokine modulation, BMF reduction, and clearance of high-risk mutations [47]. These endpoints will likely be the focus of future studies evaluating momelotinib and other therapies.
Anemia-directed therapies in development include elritercept, an investigational activin receptor type IIA ligand trap, which has shown promise in early-phase studies, including the ongoing phase 2 RESTORE trial (NCT05037760). Elritercept demonstrated improvements in hemoglobin, reductions in transfusion burden, and maintenance of platelet counts, both as monotherapy and in combination with ruxolitinib [48].
The Myelofibrosis Symptom Assessment Form version 4.0 (MFSAF v4.0), used in the MOMENTUM trial, has been validated as a reliable tool for evaluating symptom burden in MF [49]. The safety profile of momelotinib was generally comparable to other JAK inhibitors, with no significant differences in serious AEs, treatment discontinuation, or mortality. However, our analysis identified a higher incidence of dizziness (OR: 1.70; 95% CI: 1.05 - 2.74) and nausea (OR: 3.07; 95% CI: 1.82 - 5.18) associated with momelotinib. Peripheral neuropathy was also reported, particularly in the SIMPLIFY-2 trial.
Importantly, despite the baseline risk of anemia and thrombocytopenia in MF, momelotinib did not significantly exacerbate these cytopenias compared to other JAK inhibitors. This may be attributed to its inhibition of ACVR1, which regulates hepcidin and iron metabolism, providing a potential mechanism for its anemia-improving effects [50]. The MoReLife real-world analysis further supports momelotinib as an effective and safe option for heavily pretreated cytopenic MF patients [51].
While this meta-analysis focuses exclusively on momelotinib, we recognize that a broader comparison with other approved JAK inhibitors (ruxolitinib, fedratinib, pacritinib) would enhance clinical context. Momelotinib’s unique anemia benefits set it apart, but differences in toxicity, symptom control, and spleen response across agents are clinically relevant. Future network meta-analyses are needed to better inform individualized treatment decisions.
| Conclusions | ▴Top |
Momelotinib is an emerging therapeutic option for patients with MF, particularly those with anemia. It has demonstrated improvements in transfusion independence while providing spleen volume reduction comparable to ruxolitinib. However, its effect on symptom burden appears less robust than that of ruxolitinib, highlighting the need for further optimization. As the treatment landscape for MF continues to evolve, the role of momelotinib should be further investigated through studies evaluating combination therapies and long-term outcomes.
| Supplementary Material | ▴Top |
Suppl 1. Search strategy used for study screening.
Suppl 2. Risk of bias assessments for double-arm studies.
Suppl 3. Risk of bias assessments for single-arm studies.
Suppl 4. Summary characteristics of the included studies.
Suppl 5. Baseline characteristics of participants.
Suppl 6. Outcomes characteristics of the included studies.
Suppl 7. Forest plot of overall response rate.
Suppl 8. Sensitivity analyses for splenic response rate and TSS response rate.
Suppl 9. Forest plots of safety outcomes, such as any adverse event, dizziness, nausea, adverse events leading to discontinuation, and drug-related mortality.
Suppl 10. Forest plots of dose reduction and serious adverse events.
Suppl 11. Sensitivity analyses for any adverse events, discontinuation events, diarrhea, thrombocytopenia, peripheral neuropathy, and anemia.
Acknowledgments
The authors would like to acknowledge all researchers whose original studies were included in this systematic review and meta-analysis. This article is a revised and expanded version of an abstract entitled “Efficacy and Safety of Momelotinib in Myelofibrosis: A Systematic Review and Meta-Analysis with a Focus on Anemia Outcomes”, which was published online for the 65th American Society of Hematology (ASH) Annual Meeting, San Diego, CA, USA, December 7 - 10, 2024 [52].
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable. This study is a systematic review and meta-analysis of previously published data and did not involve human participants directly.
Author Contributions
Conceptualization: J.A.-N. and M.A-d. Methodology: J.A.-N. and F.A.-M. Software: M.A-d. and M.A.-Q. Validation: F.A.-M., H.K., N.A.-K., and O.E.-S. Formal analysis: M.A.-Q. and O.K. Investigation: J.A.-N. and S.H.A. Resources: M.A-d. and O.S. Data curation: J.A.-N. Writing - original draft preparation: J.A.-N. Writing - review and editing: M.A-b, T.P., and M.O.J. Visualization: M.A-d. Supervision: J.A.-N. and M.A-d. Project administration: M.A-d. Review: T.P., M.O.J., and M.A-b.
Data Availability
The data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
MF: myelofibrosis; PMF: primary myelofibrosis; SMF: secondary myelofibrosis; MPN: myeloproliferative neoplasm; AML: acute myeloid leukemia; JAK: Janus kinase; STAT: signal transducer and activator of transcription; JAK2: Janus kinase 2; MPL: myeloproliferative leukemia virus oncogene; CALR: calreticulin; Hb: hemoglobin; ACVR1: activin A receptor type 1; RCT: randomized controlled trial; TSS: total symptom score; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; CENTRAL: Cochrane Central Register of Controlled Trials; MeSH: medical subject heading; OR: odds ratio; CI: confidence interval; IWG-MRT: International Working Group-Myeloproliferative Neoplasms Research and Treatment; AE: adverse event; ECOG: Eastern Cooperative Oncology Group; BMI: body mass index; IPSS: International Prognostic Scoring System; DIPSS: Dynamic International Prognostic Scoring System; BMF: bone marrow fibrosis; TYK2: tyrosine kinase 2; FLT3: FMS-like tyrosine kinase 3; IRAK1: interleukin-1 receptor-associated kinase 1; VAF: variant allele frequency; ESAs: erythropoiesis-stimulating agents; IMiDs: immunomodulatory drugs; FDA: Food and Drug Administration; MFSAF: Myelofibrosis Symptom Assessment Form; ROBINS-I: Risk of Bias in Nonrandomized Studies of Interventions
| References | ▴Top |
- Mughal TI, Vaddi K, Sarlis NJ, Verstovsek S. Myelofibrosis-associated complications: pathogenesis, clinical manifestations, and effects on outcomes. Int J Gen Med. 2014;7:89-101.
doi pubmed - Tefferi A, Gangat N, Pardanani A, Crispino JD. Myelofibrosis: genetic characteristics and the emerging therapeutic landscape. Cancer Res. 2022;82(5):749-763.
doi pubmed - Quintas-Cardama A, Kantarjian H, Pierce S, Cortes J, Verstovsek S. Prognostic model to identify patients with myelofibrosis at the highest risk of transformation to acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2013;13(3):315-318.e312.
doi pubmed - Tremblay D, Schwartz M, Bakst R, Patel R, Schiano T, Kremyanskaya M, Hoffman R, et al. Modern management of splenomegaly in patients with myelofibrosis. Ann Hematol. 2020;99(7):1441-1451.
doi pubmed - Bose P, Verstovsek S. Management of myelofibrosis after ruxolitinib failure. Leuk Lymphoma. 2020;61(8):1797-1809.
doi pubmed - Sastow D, Tremblay D. Emerging treatment options for myelofibrosis: focus on anemia. Ther Clin Risk Manag. 2023;19:535-547.
doi pubmed - Tefferi A, Pardanani A, Gangat N. Momelotinib (JAK1/JAK2/ACVR1 inhibitor): mechanism of action, clinical trial reports, and therapeutic prospects beyond myelofibrosis. Haematologica. 2023;108(11):2919-2932.
doi pubmed - Bose P. Momelotinib for the treatment of myelofibrosis. Blood. 2024;144(7):708-713.
doi pubmed - Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-34.
doi pubmed - Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions, version 6.5 (updated August 2024); Cochrane, 2024. Available online: www.training.cochrane.org/handbook.
- Barbui T, Thiele J, Vannucchi AM, Tefferi A. Rationale for revision and proposed changes of the WHO diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis. Blood Cancer J. 2015;5(8):e337.
doi pubmed - Mesa RA, Schwager S, Radia D, Cheville A, Hussein K, Niblack J, Pardanani AD, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199-1203.
doi pubmed - Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, te Boekhorst PA, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098-4103.
doi pubmed - Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT, Verstovsek S, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5):1497-1503.
doi pubmed - Le-Rademacher JG, Hillman S, Storrick E, Mahoney MR, Thall PF, Jatoi A, Mandrekar SJ. Adverse event burden score-A versatile summary measure for cancer clinical trials. Cancers (Basel). 2020;12(11):3251.
doi pubmed - Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
doi pubmed - Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
doi pubmed - Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002.
doi pubmed - Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395-411.
doi pubmed - Pardanani A, Laborde RR, Lasho TL, Finke C, Begna K, Al-Kali A, Hogan WJ, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322-1327.
doi pubmed - Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, Passamonti F, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5(2):e73-e81.
doi pubmed - Mesa RA, Kiladjian JJ, Catalano JV, Devos T, Egyed M, Hellmann A, McLornan D, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in janus kinase inhibitor-naive patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850.
doi pubmed - Verstovsek S, Gerds AT, Vannucchi AM, Al-Ali HK, Lavie D, Kuykendall AT, Grosicki S, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet. 2023;401(10373):269-280.
doi pubmed - Gupta V, Mesa RA, Deininger MW, Rivera CE, Sirhan S, Brachmann CB, Collins H, et al. A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica. 2017;102(1):94-102.
doi pubmed - Pardanani A, Gotlib J, Roberts AW, Wadleigh M, Sirhan S, Kawashima J, Maltzman JA, et al. Long-term efficacy and safety of momelotinib, a JAK1 and JAK2 inhibitor, for the treatment of myelofibrosis. Leukemia. 2018;32(4):1035-1038.
doi pubmed - Oh ST, Talpaz M, Gerds AT, Gupta V, Verstovsek S, Mesa R, Miller CB, et al. ACVR1/JAK1/JAK2 inhibitor momelotinib reverses transfusion dependency and suppresses hepcidin in myelofibrosis phase 2 trial. Blood Adv. 2020;4(18):4282-4291.
doi pubmed - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405.
doi pubmed - Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2021;96(1):145-162.
doi pubmed - Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008-3014.
doi pubmed - Chifotides HT, Bose P, Verstovsek S. Momelotinib: an emerging treatment for myelofibrosis patients with anemia. J Hematol Oncol. 2022;15(1):7.
doi pubmed - Shawky AM, Almalki FA, Abdalla AN, Abdelazeem AH, Gouda AM. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics. 2022;14(5):1001.
doi pubmed - Saeed I, McLornan D, Harrison CN. Managing side effects of JAK inhibitors for myelofibrosis in clinical practice. Expert Rev Hematol. 2017;10(7):617-625.
doi pubmed - Talpaz M, Kiladjian JJ. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia. 2021;35(1):1-17.
doi pubmed - Mascarenhas J. Pacritinib for the treatment of patients with myelofibrosis and thrombocytopenia. Expert Rev Hematol. 2022;15(8):671-684.
doi pubmed - Verstovsek S, Mesa R, Gupta V, Lavie D, Dubruille V, Cambier N, Platzbecker U, et al. Momelotinib long-term safety and survival in myelofibrosis: integrated analysis of phase 3 randomized controlled trials. Blood Adv. 2023;7(14):3582-3591.
doi pubmed - Gupta V, Oh S, Devos T, Dubruille V, Catalano J, Somervaille TCP, Platzbecker U, et al. Momelotinib vs. ruxolitinib in myelofibrosis patient subgroups by baseline hemoglobin levels in the SIMPLIFY-1 trial. Leuk Lymphoma. 2024;65(7):965-977.
doi pubmed - Harrison CN, Vannucchi AM, Recher C, Passamonti F, Gerds AT, Hernandez-Boluda JC, Yacoub A, et al. Momelotinib versus continued ruxolitinib or best available therapy in JAK inhibitor-experienced patients with myelofibrosis and anemia: subgroup analysis of SIMPLIFY-2. Adv Ther. 2024;41(9):3722-3735.
doi pubmed - Oh ST, Verstovsek S, Gupta V, Platzbecker U, Devos T, Kiladjian JJ, McLornan DP, et al. Changes in bone marrow fibrosis during momelotinib or ruxolitinib therapy do not correlate with efficacy outcomes in patients with myelofibrosis. EJHaem. 2024;5(1):105-116.
doi pubmed - Harrison CN, Mesa R, Talpaz M, Gupta V, Gerds AT, Perkins A, Goh YT, et al. Longitudinal assessment of transfusion intensity in patients with JAK inhibitor-naive or -experienced myelofibrosis treated with momelotinib. Clin Lymphoma Myeloma Leuk. 2025;25(3):199-211.
doi pubmed - Li Y, Zhu S, Liu W, Ming J, Wang X, Hu X. Ruxolitinib-based combinations in the treatment of myelofibrosis: worth looking forward to. Ann Hematol. 2020;99(6):1161-1176.
doi pubmed - McMullin MF, Harrison CN, Niederwieser D, Demuynck H, Jakel N, Gopalakrishna P, McQuitty M, et al. The use of erythropoiesis-stimulating agents with ruxolitinib in patients with myelofibrosis in COMFORT-II: an open-label, phase 3 study assessing efficacy and safety of ruxolitinib versus best available therapy in the treatment of myelofibrosis. Exp Hematol Oncol. 2015;4:26.
doi pubmed - Masarova L, Verstovsek S, Hidalgo-Lopez JE, Pemmaraju N, Bose P, Estrov Z, Jabbour EJ, et al. A phase 2 study of ruxolitinib in combination with azacitidine in patients with myelofibrosis. Blood. 2018;132(16):1664-1674.
doi pubmed - Mascarenhas JO, Rampal RK, Kosiorek HE, Bhave R, Hexner E, Wang ES, Gerds A, et al. Phase 2 study of ruxolitinib and decitabine in patients with myeloproliferative neoplasm in accelerated and blast phase. Blood Adv. 2020;4(20):5246-5256.
doi pubmed - Bewersdorf JP, Giri S, Wang R, Podoltsev N, Williams RT, Rampal RK, Tallman MS, et al. Interferon therapy in myelofibrosis: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2020;20(10):e712-e723.
doi pubmed - Holmstrom MO, Andersen M, Traynor S, Ahmad SM, Lisle TL, Handlos Grauslund J, Skov V, et al. Therapeutic cancer vaccination against mutant calreticulin in myeloproliferative neoplasms induces expansion of specific T cells in the periphery but specific T cells fail to enrich in the bone marrow. Front Immunol. 2023;14:1240678.
doi pubmed - Passamonti F, Harrison CN, Mesa RA, Kiladjian JJ, Vannucchi AM, Verstovsek S. Anemia in myelofibrosis: current and emerging treatment options. Crit Rev Oncol Hematol. 2022;180:103862.
doi pubmed - Pemmaraju N, Verstovsek S, Mesa R, Gupta V, Garcia JS, Scandura JM, Oh ST, et al. Defining disease modification in myelofibrosis in the era of targeted therapy. Cancer. 2022;128(13):2420-2432.
doi pubmed - Harrison C, Chee LCY, Devos T, Fox ML, Iurlo A, Palandri F, Ross DM, et al. Hematological improvement and other clinical benefits of elritercept as monotherapy and in combination with ruxolitinib in participants with myelofibrosis from the ongoing phase 2 RESTORE trial. Blood. 2024;144(Suppl 1):997.
doi - Daskalopoulou C, Gorsh B, Dumi G, Deheshi S, Gwaltney C, Paty J, Ellis C, et al. Myelofibrosis symptom assessment form total symptom score version 4.0: measurement properties from the MOMENTUM phase 3 study. Qual Life Res. 2025;34(3):739-750.
doi pubmed - Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, Seifert M, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production, and ameliorates anemia of chronic disease in rodents. Blood. 2017;129(13):1823-1830.
doi pubmed - Jilg S, Schwaab J, Sockel K, Crodel CC, Brueckl V, Stegelmann F, Jentzsch M, et al. MoReLife - real-life data support the potential of momelotinib as a safe and effective treatment option for cytopenic myelofibrosis patients. Ann Hematol. 2024;103(10):4065-4077.
doi pubmed - Aldalal’Ah M, Al-Nusair J, Al-Khateeb N, Alqudah M, El-shatel O, AlMalkawi FM, Khayyat O, et al. Efficacy and safety of momelotinib in myelofibrosis: a systematic review and meta-analysis with a focus on anemia outcomes. Blood. 2024;144(Suppl 1):6652.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.