Efficacy and Safety of Momelotinib in Myelofibrosis: A Systematic Review and Meta-Analysis With a Focus on Anemia Outcomes
DOI:
https://doi.org/10.14740/jh2094Keywords:
Myelofibrosis, Momelotinib, Anemia, Myeloproliferative neoplasmsAbstract
Background: Myelofibrosis (MF) can be primary (PMF) or secondary (SMF), with PMF driven by Janus kinases-signal transducer and activator of transcription proteins (JAK-STAT) pathway activation due to Janus kinase 2 (JAK2), the thrombopoietin receptor gene (myeloproliferative leukemia virus oncogene (MPL)), or calreticulin (CALR) mutations. Nearly 50% of PMF patients experience anemia (hemoglobin (Hb) < 10 g/dL), often worsened by JAK inhibitors like ruxolitinib and fedratinib. Momelotinib, an oral ACVR1, JAK1, and JAK2 inhibitor, improves anemia, symptoms, and splenomegaly, likely through hepcidin regulation. This review evaluates its efficacy and safety, with a focus on anemia.
Methods: A systematic review and meta-analysis were conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, including randomized controlled trials (RCTs) and clinical studies assessing momelotinib’s efficacy and safety. Primary outcomes included spleen volume reduction (≥ 35%) and anemia response (transfusion independence). Secondary endpoints included symptom burden reduction and safety.
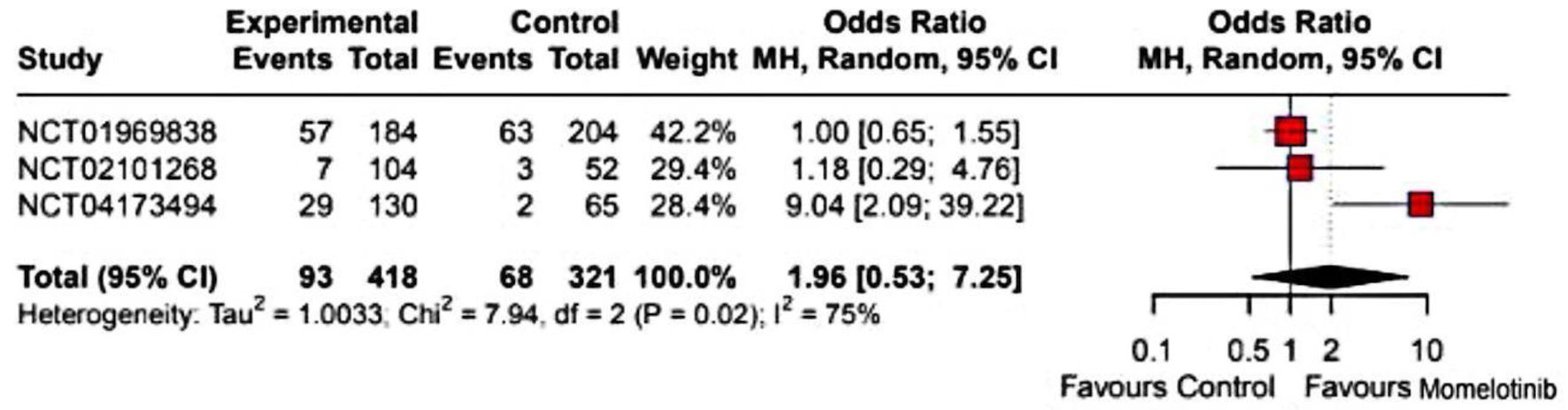
Results: Six studies, including three RCTs, met inclusion criteria. Meta-analysis showed momelotinib was noninferior to ruxolitinib in spleen volume reduction but superior in anemia benefits, increasing transfusion independence (odds ratio (OR): 2.09; 95% confidence interval (CI): 1.53 - 2.85) and reducing transfusion dependence (OR: 0.62; 95% CI: 0.45 - 0.84). Symptom burden reduction was comparable to other JAK inhibitors. Common adverse events included dizziness (OR: 1.70; 95% CI: 1.05 - 2.74) and nausea (OR: 3.07; 95% CI: 1.82 - 5.18), with no significant increase in serious adverse events.
Conclusions: Momelotinib improved anemia-related outcomes and quality of life in MF without increased adverse events. However, heterogeneity in control groups limited direct efficacy comparisons. Larger studies are needed to confirm its effectiveness and safety.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








