| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 1, February 2025, pages 1-13
Prevalence of Viral Infections and Serious Complications in Pediatric Hematopoietic Stem Cell Transplant Patients: A Ten-Year Single-Institution Retrospective Study
Calvin E. Laua, d, David J. DiTullioa, d, Holly Wilhalmeb, LaVette Bowlesc, Theodore B. Moorec, Satiro N. De Oliveirac, e
aDavid Geffen School of Medicine at UCLA, Los Angeles, CA, USA
bDepartment of Medicine Statistics Core, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
cDepartment of Pediatrics, Division of Hematology/Oncology, David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
dThese authors contributed equally to this work.
eCorresponding Author: Satiro De Oliveira, Department of Pediatrics, David Geffen School of Medicine at UCLA, Los Angeles, CA 90095-1752, USA
Manuscript submitted October 28, 2024, accepted January 23, 2025, published online February 4, 2025
Short title: Viral Infections and Complications in pHSCT
doi: https://doi.org/10.14740/jh1376
| Abstract | ▴Top |
Background: Pediatric hematopoietic stem cell transplant (pHSCT) patients are at risk for many life-threatening post-transplant complications, notably relapse, graft-versus-host disease (GvHD), and infection.
Methods: This retrospective study reviewed 10 years of pHSCT at a single institution, assessing for risk factors for post-transplantation viral infections (herpes simplex virus (HSV), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), human herpes virus 6 (HHV6), adenovirus (ADNV), and human polyoma virus 1 (BK virus)), and characterizing adverse infectious outcomes.
Results: Overall, 139 patients received 151 transplants. With respect to graft source: 73 (48.3%) were bone marrow, 67 (44.4%) umbilical cord blood (UCB), and 11 (7.2%) peripheral blood stem cells (PBSCs). Forty-one deaths occurred, for an overall mortality rate of 29.5%. The overall incidence of post-transplant viral infections was 47.7% (n = 72). Incidence of post-transplant infection varied by virus type: 3.97% HSV, 0.67% VZV, 3.97% EBV, 24.5% CMV, 14.5% HHV6, 12.6% ADNV, and 12.6% BK virus. Viral encephalitis, though relatively uncommon, was primarily caused by HHV6 and more common in UCB transplants. Overall, cell source and donor source were identified with statistically significant correlation to both risk of infection and mortality.
Conclusions: Post-transplant viral infection remains as a serious adverse event in pediatric patients, and thus prospective studies should be performed to implement early intervention and more aggressive treatment in select high-risk patients. More studies specifically addressing infection risks in cord blood transplants and risk factors for post-transplant viral encephalitis are warranted.
Keywords: Pediatric hematopoietic stem cell transplant; Viral infection; Viral reactivation; Herpesvirus
| Introduction | ▴Top |
Hematopoietic stem cell transplantation (HSCT) is utilized as a potentially curative therapy for many types of childhood diseases, including malignancies, hemoglobinopathies, primary immune deficiencies, inborn errors of metabolism, and marrow failure syndromes. In the peri- and post-transplant periods, an HSCT patient may encounter any number of serious transplant-related complications, such as engraftment syndrome, acute graft-versus-host disease (aGvHD), organ failure, and most notably, bacterial/fungal/viral infections. Viral infection and reactivation remain as major contributors to short- and long-term morbidity and mortality in pediatric HSCT (pHSCT) patients. In this population, the incidence of viral infection in allogeneic HSCT is estimated to be 55-89% [1-6].
In the pre-transplant conditioning phase, high doses of radiation, chemotherapy, and serotherapy are utilized to eliminate residual disease, creating a niche for the arriving graft and prevent graft rejection. In the post-transplant period, immunosuppressants, most commonly corticosteroids and calcineurin/mammalian target of rapamycin (mTOR) inhibitors, are given as prophylaxis against engraftment syndrome and aGvHD. This profound immunosuppression leaves patients susceptible to infection in addition to the nascent immune reconstitution [7, 8].
The herpesviruses are the main pathogens contributing to viral infection in the post-transplant period. Herpes simplex virus (HSV), cytomegalovirus (CMV), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), human herpes virus 6 (HHV6), and adenovirus (ADNV) are all commonly acquired in childhood, establish latency within the host, and remain suppressed by a healthy immune system. In the periods of profound immunosuppression and dysfunction, these viruses can reactivate and cause serious complications including hepatitis, colitis, pneumonitis, encephalitis, marrow suppression, and secondary malignancy [3, 9]. Moreover, peri-transplant viral reactivations can trigger serious HSCT complications, such as GvHD and graft failure. These reactivations can even have a negative effect on short- and long-term immune reconstitution [7]. Both prophylaxis and treatment with antivirals such as acyclovir, ganciclovir, cidofovir, and foscarnet have been utilized for the management of viral reactivations. HSV and CMV seropositive pediatric patients commonly receive acyclovir, ganciclovir or letermovir prophylaxis, respectively. Cidofovir and foscarnet are generally reserved for treating severe/symptomatic viremia. The utilization of these medications presents their own risks, including nephrotoxicity, hepatotoxicity, and marrow toxicity.
This 10-year, single-center retrospective study evaluates the rates of viral reactivation across several epidemiologic and clinical outcome variables. The goal of this work was to characterize post-transplant viral reactivations in the pHSCT population, and to identify clinical risk factors for the development of serious viral infection or reactivation to better guide and tailor viral surveillance, treatment, and management in this population.
| Materials and Methods | ▴Top |
We conducted a retrospective assessment of all pediatric stem cell transplants performed at UCLA Mattel Children’s Hospital from January 2008 to December 2017.
Viral infections or reactivations, antiviral prophylaxis and treatment were documented. More detailed analyses were performed of patients who died, with regard to causes of death as well as any contributing active viral infections at time of death. Patients who received any autologous transplants were excluded from analysis, and so were those who were cared for outside of UCLA during the immediate post-transplant period. Transplant follow-up time was defined as the period from the day of transplant until a full year of follow-up for the last transplanted patient in the cohort.
Transplantation methods
Conditioning regimens varied based on primary diagnosis, disease status, and other individual factors. Cell sources included umbilical cord blood (UCB), peripheral blood stem cells (PBSCs), and bone marrow (BM). Donor sources were classified as autologous, matched related, matched unrelated, and related haploidentical donors. The standard approach at UCLA Pediatrics has been to perform myeloablative conditioning regimen for malignancies or genetic disorders, excluding juvenile myelomonocytic leukemia (JMML) and hemophagocytic lymphohistiocytosis (HLH). Preference was generally given to the use of BM as a cell source, to decrease risk of aGvHD and favor engraftment, even for patients receiving human leukocyte antigen (HLA)-matched sibling donor or related haploidentical transplants. UCB was considered for patients for whom a minimal cell dose of 3.5 - 5.0 × 107 total nucleated cell (TNC)/kg could be assured. Mobilized PBSCs were used as last option, in the case of reluctant or smaller/younger donors. GvHD prophylaxis followed institutional protocols for all transplant patients in the study period. Recipients of UCB transplants received cyclosporine and steroid (methylprednisolone or prednisolone at 0.5 mg/kg/day). Recipients of sibling donor transplants received single drug prophylaxis with tacrolimus. Recipients of matched unrelated donor grafts (BM or PBSC) received two-drug prophylaxis with tacrolimus and methotrexate 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6 and +11. Recipients of haploidentical transplants received tacrolimus and mycophenolate mofetil. Time to engraftment was divided into three periods (under 28 days, 28 - 42 days, longer than 42 days) or graft failure, defined as a failure to achieve an absolute neutrophil count (ANC) > 200 × 106/L by day +28.
Viral screening and prophylaxis
Pre-transplant screening included recipient serologies for CMV, HSV, EBV, HIV, and hepatitis viruses. VZV, HHV6, BK, and ADNV were not routinely screened prior to transplant in the study period, as there are no standard prophylaxis measures to prevent infection. Per institutional standard operating procedures, prophylaxis should be administered to all patients seropositive for CMV and HSV, consisting of ganciclovir 6 mg/kg over days -7 to -2, for CMV seropositive patients, and acyclovir 10 - 20 mg/kg/dose BID or TID from day 0 to +100, for HSV seropositive patients. Letermovir was not used at our institution for CMV prophylaxis in the study period. Twice-a-week polymerase chain reaction (PCR) viral titers in blood for CMV and EBV were performed for all transplant recipients. PCR and antibody studies for ADNV (blood, stools, and cerebrospinal fluid (CSF)), BK virus (blood and urine), VZV (blood), HHV6 (blood and CSF), and respiratory viruses (nasopharyngeal aspirate) were performed in symptomatic patients at the physicians’ discretion. Viral infections were defined by detection of antibody titers > 1:20 or PCR > 500 copies/mL, with or without signs or symptoms. Viral reactivations were defined by rising PCR titers in patients with positive pre-transplant serology. CMV disease was identified by rising viremia titers in symptomatic patients, or biopsy-proven tissue specimens. Viral infections were reported at the first documented date of viremia, divided into occurrence from days 0 - 30, 31 - 100, or > 101 post-transplantation phases.
Statistical analysis
Patient demographic and clinical characteristics were summarized using frequencies and percentages. To determine factors associated with infection post-transplant, a mixed effects logistic regression model was used with a fixed effect for each patient factor individually with a random effect for patient to account for multiple transplant events. To assess factors that were associated with survival after transplant, a Fine-Grey survival model was used assessing time to death post-first transplant with a competing event for a transplant, assuming that an additional transplant modifies the hazard of survival. A multivariate survival model was then constructed using variables which were significant in the univariate analyses of infection and time to death as well as variables of clinical interest. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC). P values < 0.05 were considered statistically significant.
Ethical compliance with human/animal study
All information was collected from the medical records. Informed consent for data collection and analysis was routinely obtained from all patients receiving HSCT at UCLA, according to a protocol approved by the Institutional Review Board (IRB), following the standards set by the Center for International Blood and Marrow Transplant Research (CIBMTR).
| Results | ▴Top |
Patient characteristics
We report 151 transplants in 139 patients (median age of 9.58, range 0 - 22) performed on the study period. Patient characteristics are summarized in Table 1. Median age at transplant was 9 years and the median follow-up time was 853 days (range 0 - 4,022 days). With respect to graft source, 73 (48.3%) used BM, 67 (44.4%) UCB, and 11 (7.2%) PBSCs. In terms of HLA-match status, 46 (30.4%) were from matched related donors, 95 (62.3%) were from matched unrelated donors, and 10 (6.6%) were from related haploidentical. In the study period, all haploidentical transplants were from related donors. Most transplants were conducted to treat malignant diagnoses (n = 99, 65.5%), with the most common malignancy being acute leukemia (n = 86, 56.9%), followed by chronic leukemia (n = 17, 11.3%), and lymphoma (n = 4, 2.6%). Of the non-malignant diagnosis, primary immunodeficiencies (n = 17, 11.3%) and bone marrow failure syndromes (n = 17, 11.3%) were the most common.
 Click to view | Table 1. Demographics of Study Patients |
Outcomes
One hundred and twelve transplants (74.1%) successfully engrafted neutrophils before day +28. Engraftment failure occurred in 15 (9.9%) of cases; five of such failures were rescued by a second transplant within 30 days. Thirty-three of 67 (49.3%) UCB patients and 39 of 73 (53.4%) marrow patients developed viral infections.
Viral infections: a broad overview
Viral infection occurred in 72 post-transplant courses (48.3%). Among the three post-transplantation phases, there were 46 infections from post-transplant day 0 - 30, 49 infections from day 31- 100, and 15 infections from day > 101 (Fig. 1). Incidence of initial viral infection in each of the post-transplantation phases was calculated as follows. Thirty-five initial infections were documented in post-transplantation days +0 to 30 (incidence 25.2%). In the day +31 to 100 phase, 29 initial infections were documented (incidence 21.0%, cumulative incidence 28.1%, and prevalence 46.4%). In the post-transplant day +100 phase, three initial infections were documented (incidence 2.48%, cumulative incidence 5.26%, and prevalence 55.4%) (Fig. 1). Overall, across 139 first transplant patients, 67 experienced a viral infection, for an overall incidence of 48.2%. In 30 cases (19.9%), the patients developed multiple viral infections: 23 patients experienced two infections, six patients experienced three infections, and one patient experienced four infections. The overall incidence of specific viral infection is as follows: HSV (3.97%), VZV (0.67%), EBV (3.97%), CMV (24.5%), HHV6 (14.5%), BK (12.6%). aGvHD occurred in 48.9% of patients, with grades 1 - 2 occurring in 25.9% and grades 3 - 4 occurring in 23%. There was no association between GvHD and viral infection (P = 0.2737) or GvHD grading and viral infection (P = 0.4051).
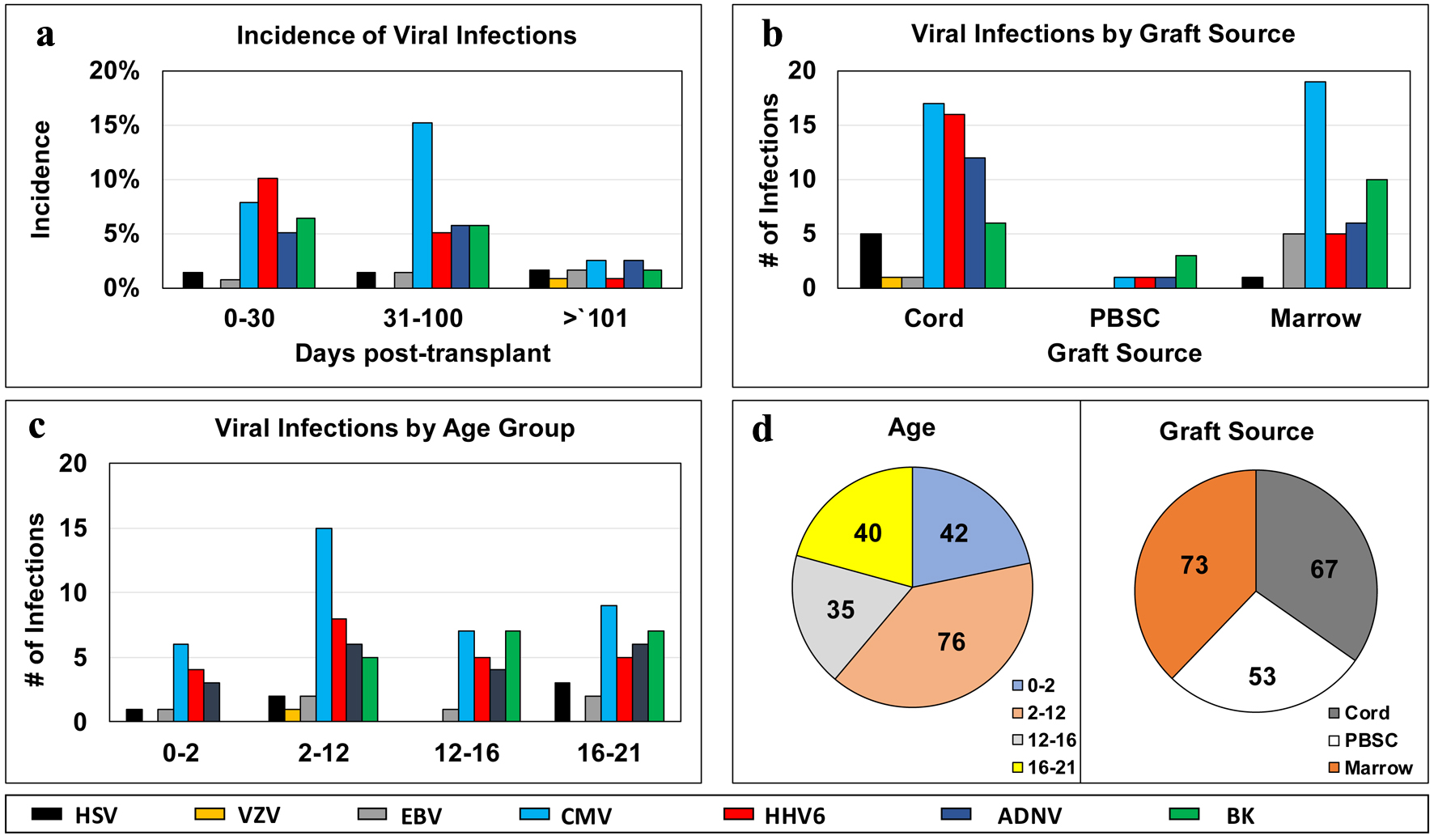 Click for large image | Figure 1. Distribution of infectious episodes by virus, along post-transplant phases (a), graft sources (b), age groups (c); and pie charts of distribution of viral infection episodes by age groups or graft sources (d). ADNV: adenovirus; BK virus: human polyoma virus 1; BM: bone marrow; CMV: cytomegalovirus; EBV: Epstein-Barr virus; GvHD: graft-versus-host disease; HHV6: human herpes virus 6; HSV: herpes simplex virus; PBSC: peripheral blood stem cell; pHSCT: pediatric hematopoietic stem cell transplant; UCB: umbilical cord blood; VZV: varicella-zoster virus. |
Forty-one deaths occurred in 139 patients, with 60.5% of patient deaths with GvHD, and viral infection was present in 62.8% of all deaths. There was no statistical correlation between viral infections at death and presence of aGvHD (P = 0.6634) or viral infections at death and GvHD grading (P = 0.6954).
HSV infection
Pre-transplant serology demonstrated 81 seropositive patients, for an HSV prevalence of 53.6% (Table 1). With respect to prophylaxis, 75 of 81 seropositive cases received pre-transplant acyclovir, and six did not. We documented six HSV infections (overall incidence of 3.97%); two occurred < 30 days after transplant, two occurred 31 - 100 days after transplant, and two occurred > 101 days after transplant (Fig. 1). Five of these infections (83.3%) were reactivations for pre-transplant seropositive patients, and one seronegative patient developed HSV infection late in their post-transplant course (> 101 days). Out of the 75 seropositive patients who received acyclovir prophylaxis, five (6.67%) developed an HSV reactivation, all within the first 100 days of transplant. Of the six seropositive patients who did not receive acyclovir prophylaxis, none developed HSV reactivation.
VZV infection
Varicella antibody titers were not routinely checked as part of the pre-transplant virology panel during the study period. In this retrospective study cohort, we report 28 seropositive, 12 seronegative, and 99 patients who did not receive VZV serology testing. Post-transplant VZV infection was rare in this cohort, with only one case of infection (0.67%), which occurred > 101 days after transplant in a cord transplant recipient (Fig. 1).
EBV infection
No patients who were EBV seronegative prior to transplant had EBV infections after transplantation. Of 62 EBV seropositive patients pre-HSCT, five had EBV reactivations post-transplant (8.06%). Overall incidence of EBV infection was 3.97%. Among the cases of EBV reactivation, two occurred between 31 and 100 days post-transplant and three occurred after 100 days (Fig. 1). Three cases were among BM recipients, and two were in cord blood recipients (Fig. 1). One patient with unknown pre-transplant titers developed EBV infection. Two patients with EBV reactivations ultimately died; both patients had positive EBV titers at the time of death.
CMV infection
We report a pre-transplant seropositivity rate of 53.6% (n = 81) for CMV (Table 1). There were a total of 37/151 cases (24.5%) of CMV infection. More specifically, there were 31 cases of CMV reactivation. Sixty-nine of 81 CMV seropositive patients received ganciclovir prophylaxis, of which 26/69 (37.6%) experienced CMV reactivation. Of the 12 patients who were CMV seropositive but did not receive ganciclovir prophylaxis, 5/12 patients (41.6%) developed CMV reactivation. One CMV seronegative patient who did not receive prophylaxis developed CMV infection. Twenty-seven of 50 CMV R+/D+ pairs and 22 of 30 R+/D- pairs received prophylaxis. Of patients receiving ganciclovir prophylaxis, seven patients who did not receive ganciclovir prophylaxis experienced CMV infections: two were R+/D+ pairs, three were R+/D- pairs, and two were R-/D+ pairs.
As expected, recipient serostatus was highly predictive of reactivation. Of 68 R- cases, only three had CMV infection post-transplant (4.41%), while in 80 R+ patients, 34 developed CMV infections (42.5%), for a total of 37 CMV infections (P = 1.293 × 10-4). In the R+/D+ group, reactivation occurred in 44% of cases, compared to 40% and 8.1% reactivation rates in the R+/D- and R-/D+ groups, respectively (Table 2). Most reactivations occurred between 31 and 100 days post-transplant (n = 22, 59.4%), and a substantial number occurred in the first 30 days as well (n = 12, 32.4%). Two patients had multiple distinct reactivations after the first transplant, defined by at least two negative CMV DNA PCR tests between positive tests (Table 2).
 Click to view | Table 2. CMV Viral Infections in the Post-Transplant Period |
UCB and BM graft sources had comparable rates of infection of 25.3% and 26.0%, respectively. There was one case of CMV infection in PBSC grafts. With respect to age at transplant, infection rates were comparable across the spectrum, with the 20% incidence of CMV infection in 0 - 2 years of age, 25.8% in the 2 - 12 age group, and 24.1% in the 16 - 21 group (24.1%) (Fig. 1).
There were 14 patients diagnosed with CMV-associated disease (35.9%). CMV disease occurred only in R+ cases: 23.3% of R+/D- pairs and 15% in R+/D+ pairs. Between these two groups, there was no significant difference in rates of CMV reactivation (P = 0.6320), and no significant difference in the rate of CMV disease (P = 0.2875) (Table 2). There were six deaths in patients with CMV-associated disease, with a mortality rate in patients with CMV disease of 42.9% (six deaths in 14 CMV disease cases).
HHV6 infection
HHV6 serology was not routinely checked prior to transplant. Twenty-two (14.5%) patients were diagnosed with post-transplant HHV6 infection, to whom testing was performed in the presence of persistent fevers and/or altered mental status. Fourteen cases occurred < 30 days (63.6%), seven cases occurred 31 - 100 days (31.8%), and one case occurred > 101 days post-transplant (4.5%). UCB transplants comprised 16 (72.7%) of all HHV6 infections, followed by five (22.7%) in BM, and one (4.5%) in PBSC (P = 0.01479) (Table 3).
 Click to view | Table 3. HHV6 Infections in the Post-Transplant Period |
ADNV infection
ADNV serology was not routinely checked prior to transplant. We documented 19 cases of ADNV infection (12.6%), to whom testing was performed in the presence of persistent fevers. Eight occurred < 30 days (42%), eight occurred between 31 and 100 days (42%), and three occurred > 101 days after transplant (16%).
Across all graft sources, one PBSC transplant had ADNV infection, whereas six of 73 BM transplants, and 12 of 67 of UCB transplants were affected by ADNV (P = 0.2107) (Fig. 1).
BK infection
We report 19 cases of BK viruria detected in patients with hemorrhagic cystitis (12.6%). In two cases, there was concomitant BK viremia. Symptomatic infection occurred early in the post-transplant course, with 17 of 19 cases (89.5%) diagnosed within 100 days. With respect to age, BK disproportionately affected older children, with 24.1% and 20.5% incidence in all transplants aged 12 - 16 and 16 - 21, respectively. There were no documented BK infections in the 0 - 2 age group (P = 0.01380). There was no significant difference in the distribution of BK infection among UCB, PBSC, and BM groups (P = 0.2185) (Fig. 1).
Viral encephalitis
Viral encephalitis is a serious complication in severely immunocompromised patients. We report seven cases of viral encephalitis diagnosed in the study population (4.64%). All seven cases were in malignant primary disease patients who received UCB graft. With regard to viral etiologies, four (57%) cases resulted from HHV6 and one case from ADNV, confirmed by PCR on CSF. One case presented with clinical symptoms of encephalitis and active HHV6 viremia. Another case presented with clinical encephalitis, an HSV CSF antibody titer of 1.6, with negative viral cultures and CSF. Another case was presumed to be VZV encephalitis through diagnosis of exclusion. Six out of seven patients diagnosed with viral encephalitis died (85.7%), with half of deaths occurring within the first 100 days post-transplant (Table 4).
 Click to view | Table 4. Descriptions and Outcomes of Patients Diagnosed With Viral Encephalitis |
Analysis of risk of death
Forty-one deaths occurred in 139 patients, for an overall survival of 70.5% for the median follow-up of 837 days (Fig. 2). There were no differences in survival by sex (P = 0.9636), age group (P = 0.2072) or recipient CMV status (P = 0.9818) (Fig. 2). Graft cell source had a lower survival for UCB and PBSC transplants (P = 0.0031) (Fig. 2). GvHD was present in 60.5% of patient deaths, and viral infection was present in 62.8% of all deaths. There was no statistical correlation between viral infections at death and presence of aGvHD (P = 0.6634) or viral infections at death and GvHD grading (P = 0.6954). Cancer relapse was the primary cause in 34.2% of patient deaths, while transplant-related mortality was the primary cause in 65.8%, including graft failure, GvHD, infections and other transplant-related toxicities. Eleven patients had infection as the primary cause of death, of which three patients had viral infection as the primary cause, while the other eight patients had concomitant infections of viruses, fungi and bacteria.
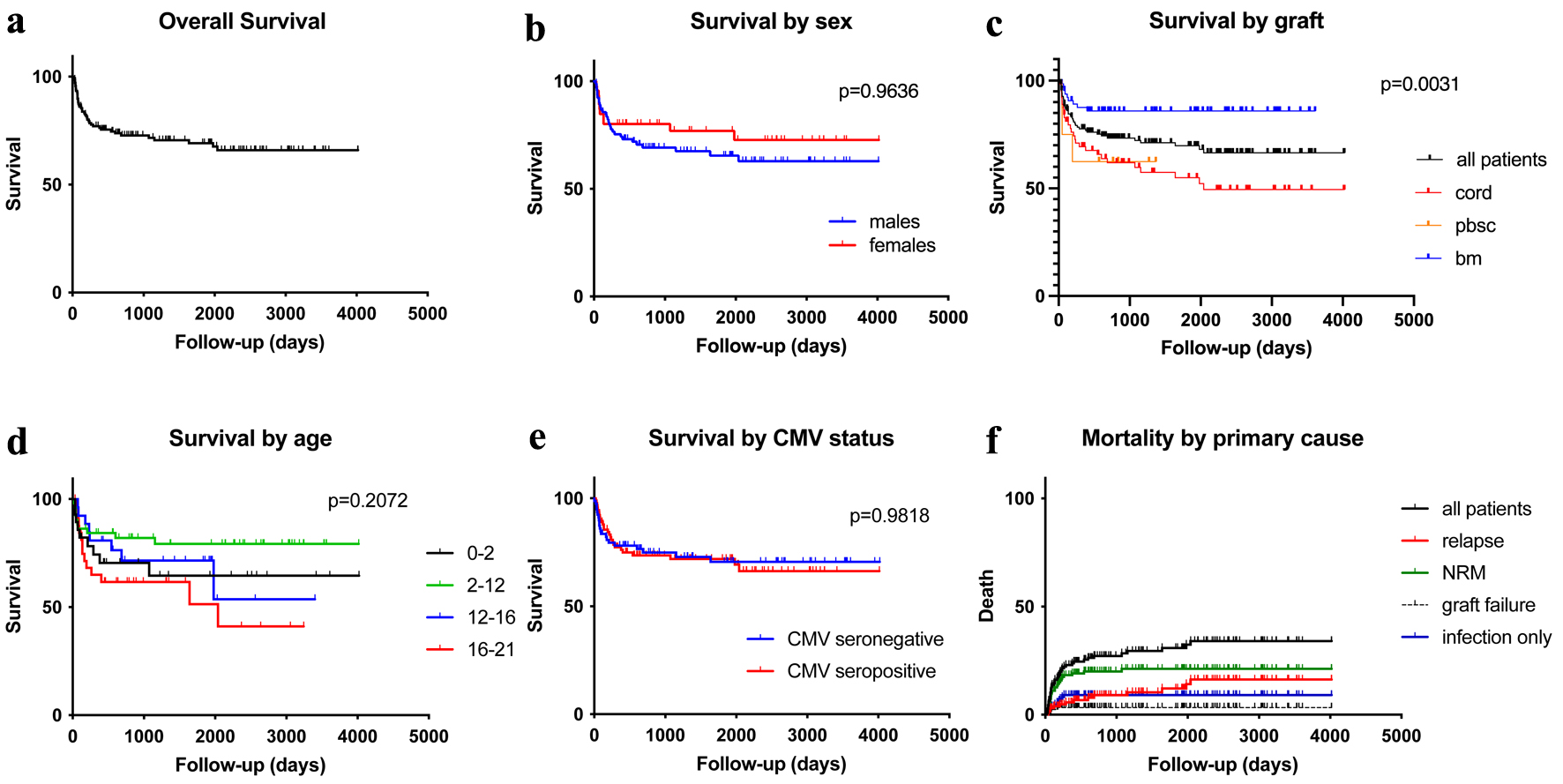 Click for large image | Figure 2. Kaplan-Meier plots for the study population: overall survival (a), overall survival by sex (b), overall survival by graft sources (c), overall survival by age groups (d), overall survival by patient’s CMV status (e), and mortality by primary cause of death (f). CMV: cytomegalovirus. |
UCB transplants comprised 70.7% (n = 29) of deaths, and 24.4% (n = 10) and 7.3% (n = 3) for BM and PBSC, respectively. The overall mortality rate by graft source was 43.3% in UCB, 13.7% in BM, and 27.3% in PBSC transplants (Fig. 2).
Malignant relapse was the leading primary cause of death (n = 14, 34.2%). GvHD was reported in 10 (24.4%) of deceased patients. Graft failure was the primary cause of death in four patients (9.8%). Veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) was the primary cause of death in two patients (4.9%) as well. As a primary cause of death, any infection (bacterial, fungal, or viral) in the absence of relapse, graft failure, SOS, or GvHD accounted for 12 deaths (29.3% of deaths, 8.6% of all transplants in the cohort); six (50%) of these deaths occurred in UCB transplants, three (25%) in BM and two (16.7%) in PBSC transplants. At the time of death, 41.5% of patients in this cohort had at least one active viral infection, 24.4% had bacterial sepsis, and 43.9% had fungal sepsis.
ADNV, CMV, and HHV6 were each present in six of 41 patients’ deaths (14.6%). Four patients had active BK infections at the time of death (9.8%). Respiratory viruses were identified in six patients at death; however, these viruses were not routinely screened and were not a primary focus of this study.
Univariate and multivariate statistical analyses of overall incidence of infections
On univariate analysis, cell source and donor source were significantly correlated to a higher risk of death, but not significantly correlated to infection. CMV status for recipient and donor was significantly correlated to a higher risk of infection, but not to a higher risk of death (Supplementary Materials 1, 2, jh.elmerpub.com). Disease status was not significantly correlated to a higher risk of death or to a higher risk of infection.
Sensitive analysis comparing random effects model using all transplant events to only first transplant event for each patient did not yield significant differences for rates of infection. It is important to remark that many other confounding factors could affect this analysis, such as cell dose, transplant recipient age, transplant recipient comorbidities and donor age, and these variables were not assessed in this study.
On a multivariate analysis level, donor source and CMV pair were found to be statistically correlated with increased risk of infection. Cell source and donor source were found to be significantly correlated with increased risk of death (Supplementary Material 3, jh.elmerpub.com).
| Discussion | ▴Top |
Overall, post-transplant viral infections and reactivations remain strong predictors of poor outcomes, from increased length of hospital stay, to development of acute GvHD, and increased mortality rate [1, 2]. More recent publications have also implicated long-term effects of survivors of viral reactivation, including cognitive delay from encephalitis, development of chronic GvHD, and altered immune reconstitution [7-9]. Previous studies have identified various risk factors for herpesvirus reactivations, such as age, donor/recipient serostatus, primary diagnosis, conditioning regimen, stem cell source, donor source, time following transplant, immune reconstitution profile, and GvHD.
Antiviral prophylaxis against HSV is highly effective. This retrospective study reports a symptomatic reactivation rate of 6.67% in patients with acyclovir prophylaxis, which is within the range found in the literature (2.6-10.2%) [8, 10, 11]. The true post-transplant prevalence of HSV is likely much higher, 23-30%, when serial oral swabs or blood samples are serially collected [10, 12, 13]. Nearly all HSV reactivations occurred within the first 100 days post-transplant, which aligns with the results of Srinivasan et al [2]. Verdeguer et al described a bimodal distribution of HSV reactivation, early on in pre-engraftment, when mucositis and profound immunosuppression are both expected, and later in the transplant course for patients treated for GvHD [9].
For CMV, in our study prophylaxis with ganciclovir achieved an overall post-transplant infection rate of 24.5%, which is well within the 13-40% rate found in the literature [1, 4, 5, 8, 12]. However, use of ganciclovir as a universal prophylactic antiviral is limited by myelosuppression. CMV prophylaxis with ganciclovir in the conditioning period was a standard protocol during the study period. Letermovir was started in use for patients aged 18 years and older at the UCLA Pediatric Hematology, Stem Cell Transplant program on November 9, 2018, after the end date of our study population timeline. Prior to institutional use of letermovir, ganciclovir was the preferred prophylaxis of choice against CMV infections at UCLA. Prior to letermovir prophylaxis, ganciclovir was found to have significant effect in reducing CMV reactivation rates in CMV+ patients, though its risk of myelosuppression in the post-transplant period was also well documented and thus was not recommended as universal prophylaxis [14, 15]. Newer agents, such as letermovir (approved in August 2024 for pediatric patients older than 6 months and weighing more than 6 kg), have demonstrated decreases in CMV-related outcomes and all-cause mortality without the myelosuppressive side effects in the adult population but prospective studies in the pediatric population are still to be completed [16]. Furthermore, due to the limited availability of letermovir in low resourced health systems on the international level, these results regarding CMV prophylaxis with ganciclovir continue to be relevant.
Our rates of CMV infection, reactivation, disease, and mortality agree with reports in the literature. Even with prophylaxis and frequent surveillance, CMV reactivation and CMV disease continue to be a major contributor to post-transplant mortality. As expected, pre-transplant recipient serology was highly predictive of CMV reactivation, with over a 10-fold increase in CMV infections in the R- vs. R+ populations. Matthes-Martin et al and Srinivasan et al postulated that negative donor serostatus is associated with CMV disease [2, 17]. In our study, there was no significant difference in rates of CMV disease in the D+ and D- groups. However, our patient population included many UCB transplants, which generally have an overall naive T-cell population that may lead to patients developing CMV disease despite their negative donor CMV serostatus. Furthermore, this study reports that nearly half of all patients with CMV disease died, indicating the severity of its diagnosis and highlighting the role of CMV as a major contributor to viral infection-related mortality.
With respect to HHV6, our data likely underreport the true incidence of reactivation, as HHV6 was not routinely screened for in the pre- and post-transplant periods. Lifetime incidence of HHV6 viremia has been described at a rate of 40-67% [1, 5]. Seventy-three percent of infections occurred in UCB transplants, which is a known risk factor for post-transplant HHV6 infection [1, 5, 8, 10, 18]. HHV6 reactivation is known to occur quite early in the post-transplant time course [10, 13], with 66.7% of reactivations occurring within 30 days, and > 99% occurring within 100 days in this study population. In our cohort, HHV6 encephalitis occurred in 3.6% of patients, which is higher than 2% described by others [10, 13, 19]. This may have resulted from a large proportion of UCB transplants, as well as this institution’s practice during the study period to provide 30 days of prednisone for engraftment syndrome prophylaxis. The rate of HHV6 encephalitis among UCB transplants has been described between 2% and 7.4% [13, 18, 19]. The increased risk of viremia and encephalitis conferred by UCB transplants is likely due to the lack of HHV6 specific immunity in antigen naive donor T cells [10] as well as increased use of steroids for engraftment syndrome or GvHD [18]. CD4+ T cells play a major role in suppressing viral reactivation [3, 7]. HHV6 targets the CD4+ T lymphocyte for viral replication, which may explain the increased likelihood for subsequent viral reactivations, encephalitis, GvHD, and increased mortality. Recent publications also point to long-term effects of HHV6 viremia on immune reconstitution and rates of GvHD [3, 7, 20]. These results demonstrate that early HHV6 reactivation is associated with encephalitis and death, which agrees with the conclusions of multiple previous studies [10, 13, 18].
Encephalitis is a feared complication of viral reactivation in immunosuppressed patients. In our cohort, we report an overall viral encephalitis incidence of 4.63%. Wu et al found a similar incidence of viral encephalitis of 6.3% in a study of similar size [11]. As expected, HHV6 was the most common viral pathogen detected in patients presenting with encephalitis and UCB transplant was a significant risk factor for HHV6 viremia and encephalitis. Mortality from viral encephalitis is high. Ogata et al reported a 58.3% overall survival at 100 days post-transplant in patients with HHV6 encephalitis, whereas patients without encephalitis had a survival rate of 80% [18]. Incorporating the analysis of HHV6 infection and viral encephalitis together, the results characterize viral encephalitis, most commonly associated with HHV6, as a major contributor to poor patient outcomes. Our data on encephalitis agree with this finding and allude to a high mortality rate for cases of encephalitis occurring within the first 30 days of transplant.
We report an infection rate of 12.6% for ADNV. Because ADNV testing was only ordered in symptomatic patients, this value underestimates the incidence of ADNV reaction of 15-50% established in the literature [1, 10, 17, 21]. While overall rates of reactivation were low, ADNV was as commonly diagnosed as HHV6 and CMV in patients who died of transplant complications. Admiraal et al demonstrated that ADNV infection is associated with lower overall survival and non-relapse mortality, which is consistent with our findings [7].
In previous studies, conditioning with total body irradiation (TBI) has been shown to confer a higher risk of delayed neutrophil engraftment, engraftment syndrome, and viral infections from CMV, EBV, and HHV6. Our study did not recapitulate that conditioning with TBI confers a higher viral infection risk in a larger study population (conditioning regimens listed in Supplementary Material 4, jh.elmerpub.com). However, our study is not powered to comment on the effect of TBI conditioning on the risk of infections. While TBI containing conditioning does present elevated post-transplant risk complications, it remains a mainstay of conditioning therapy for stem cell transplant of high-risk acute lymphoblastic leukemia (ALL), as it has a well-documented advantage on overall survival and lower risk of relapse [22-27].
We report a high mortality rate in UCB transplants when compared to other graft sources, and the rate of viral infections is a likely contributor. We report three times as many deaths caused by infection in UCB transplants than marrow transplants. UCB grafts were significantly associated with ADNV, CMV, and HHV6, the three most common viruses affecting patients who died. Other factors are likely to contribute to this finding, including higher rates of infection due to naive donor immune system and steroid use for engraftment syndrome, as well as more aggressive primary disease requiring an available donor graft in favor of waiting for the ideal donor match. Utilization of UCB cell source is, however, decreasing due to the aforementioned risks of infection and engraftment syndrome. Nevertheless, UCB remains a valuable and widely used cell source in pediatric patients, especially those with genetic disorders [19, 28].
Overall, infection was the second most common primary cause of death, behind malignancy/relapse (29.3% and 34.1%, respectively). Mortality attributed to viral illness and disease ranges from 10.9% to 24.0% in the literature [3, 17]. Determining the true impact of viral infections as a primary contributor to morality can be challenging, as viral infections commonly follow immunosuppression from GvHD, graft failure, or disease relapse. In this study, 41.5% patients had an active viral infection at the time of death. The true impact of viral reactivation and infections to mortality is underestimated by only looking at infection as a primary cause of death.
Limitations
This study is limited by its retrospective design and reliance on electronic charting notes for data collection. At our institution, the care team and policies remained mostly the same in the study period, and the statistical analysis confirms that the year of transplant was not associated with outcomes. Moreover, this study did not describe the clinical presentation and symptomatology for post-transplant viral infections, instead, it focuses on determining associations between transplant demographics and rates of viral reactivation and outcomes. While viruses such as HSV, CMV, and EBV are routinely screened in the pre- and post-transplant time period, other viruses such as ADNV, HHV6, BK, and VZV are not. Viruses that are routinely screened were well studied in the context of transplant and have established prophylaxis and/or treatments. BK, ADNV, HHV6, and VZV are not commonly screened in the post-transplant period due to their high prevalence in the general population and the paucity of clinical studies and available interventions. Therefore, these viruses are tested in serum, urine, stool, or CSF at the clinical provider discretion when the patient is presenting with symptoms, increasing pre-test probability of sampling, such as testing BK virus in cases of hematuria, or HHV6, ADNV in cases of persistent fever in absence of other sources or meningitis/encephalitis. This practice can, however, lead to misleading correlations between presence of virus and level of illness. If a patient has prolonged fevers in their post-transplant course, clinicians may test for and detect ADNV in the serum. It would be unclear, then, how much of the potential subsequent poor outcomes are caused by ADNV or whether ADNV positivity is simply a result of an overall poor engraftment response or heavy immunosuppression.
Notwithstanding, as not all viruses were routinely screened pre- or post-transplant, our study likely underestimates the true incidence and prevalence of ADNV, HHV6, VZV, and BK infections. This limitation will extend to all retrospective chart studies that draw on clinician-guided management, in the absence of prospective trials or standard institutionalized protocols. Although we do not expect significant differences in the supportive care and expertise level at this single institution during the decade of data analysis, the access to precise, high-throughput and quick turnover diagnostic testing for viral infections cannot be ignored, and most likely has impacted the quality of patient care in HSCT over the years.
Conclusions
Our large cohort, single-institution, retrospective study demonstrates that viral infections, namely CMV, HHV6, and HHV6, pose a major threat to pHSCT patients in the immediate post-transplant period. We have characterized the general timeline of infection/reactivation for these viruses in our cohort, and have determined that patient demographics, pre-transplant serology, antiviral prophylaxis, and graft source are relevant risk factors for viral reactivation. The aforementioned findings can be utilized in clinical practice guidelines for viral surveillance, as understanding the incidence of certain viral infections in the post-transplant course can improve resource utilization. In highlighting poor outcomes associated with CMV disease and viral encephalitis, this study supports early surveillance and treatment of high-risk patients.
| Supplementary Material | ▴Top |
Suppl 1. Descriptive summary of patient factors by infection event level, using random effects model.
Suppl 2. Bivariate analysis of time to death, first transplant only.
Suppl 3. Multivariate model of time to death, first transplant only.
Suppl 4. List of conditioning regimens.
Acknowledgments
None to declare.
Financial Disclosure
This study received financial support from UCLA Children’s Discovery and Innovation Institute, UCLA Jonsson Comprehensive Cancer Center, Hyundai Hope on Wheels, and American Society of Hematology Scholar Award.
Conflict of Interest
The authors declare that they have no competing interests nor conflict of interest.
Informed Consent
Informed consent for data collection and analysis was routinely obtained from all patients receiving HSCT at UCLA, according to a protocol approved by the Institutional Review Board (IRB), following the standards set by the Center for International Blood and Marrow Transplant Research (CIBMTR).
Author Contributions
DD, CL, SO and TM designed the research study. TM, SO and LB took care of patients and generated the clinical data. DD, CL, and SO reviewed and collected research data. CL and SO performed the data analysis. HW performed the univariate and multivariate statistical analyses. CL and SO were the major contributors in writing the manuscript. TM assisted in manuscript review. All authors read and approved the final manuscript.
Data Availability
The datasets generated and/or analyzed in this current study are not publicly available due to confidentiality of medical information but are available from the corresponding author on reasonable request.
Abbreviations
ADNV: adenovirus; BK virus: human polyoma virus 1; BM: bone marrow; CMV: cytomegalovirus; EBV: Epstein-Barr virus; GvHD: graft-versus-host disease; HHV6: human herpes virus 6; HSV: herpes simplex virus; PBSC: peripheral blood stem cell; pHSCT: pediatric hematopoietic stem cell transplant; UCB: umbilical cord blood; VZV: varicella-zoster virus
| References | ▴Top |
- Tsoumakas K, Giamaiou K, Goussetis E, Graphakos S, Kossyvakis A, Horefti E, Mentis A, et al. Epidemiology of viral infections among children undergoing hematopoietic stem cell transplant: a prospective single-center study. Transpl Infect Dis. 2019;21(4):e13095.
doi pubmed - Srinivasan A, Wang C, Srivastava DK, Burnette K, Shenep JL, Leung W, Hayden RT. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(1):94-101.
doi pubmed pmc - Hiwarkar P, Gaspar HB, Gilmour K, Jagani M, Chiesa R, Bennett-Rees N, Breuer J, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48(6):803-808.
doi pubmed - Fisher BT, Alexander S, Dvorak CC, Zaoutis TE, Zerr DM, Sung L. Epidemiology and potential preventative measures for viral infections in children with malignancy and those undergoing hematopoietic cell transplantation. Pediatr Blood Cancer. 2012;59(1):11-15.
doi pubmed pmc - Olkinuora HA, Taskinen MH, Saarinen-Pihkala UM, Vettenranta KK. Multiple viral infections post-hematopoietic stem cell transplantation are linked to the appearance of chronic GVHD among pediatric recipients of allogeneic grafts. Pediatr Transplant. 2010;14(2):242-248.
doi pubmed - Maltezou HC, Kafetzis DA, Abisaid D, Mantzouranis EC, Chan KW, Rolston KV. Viral infections in children undergoing hematopoietic stem cell transplant. Pediatr Infect Dis J. 2000;19(4):307-312.
doi pubmed - Admiraal R, de Koning CCH, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB, Wolfs TFW, et al. Viral reactivations and associated outcomes in the context of immune reconstitution after pediatric hematopoietic cell transplantation. J Allergy Clin Immunol. 2017;140(6):1643-1650.e9.
doi pubmed - Duver F, Weissbrich B, Eyrich M, Wolfl M, Schlegel PG, Wiegering V. Viral reactivations following hematopoietic stem cell transplantation in pediatric patients - a single center 11-year analysis. PLoS One. 2020;15(2):e0228451.
doi pubmed pmc - Verdeguer A, de Heredia CD, Gonzalez M, Martinez AM, Fernandez-Navarro JM, Perez-Hurtado JM, Badell I, et al. Observational prospective study of viral infections in children undergoing allogeneic hematopoietic cell transplantation: a 3-year GETMON experience. Bone Marrow Transplant. 2011;46(1):119-124.
doi pubmed - Costa ALF, Santos BA, Torregrossa VR, Miranda ECM, Vigorito AC, Palmieri M, Ricardo ALF, et al. Oral shedding of CMV and HSV-1 in hematopoietic stem cell transplantation patients. Oral Dis. 2021;27(6):1572-1579.
doi pubmed - Wu JL, Ma HY, Lu CY, Chen JM, Lee PI, Jou ST, Yang YL, et al. Risk factors and outcomes of cytomegalovirus viremia in pediatric hematopoietic stem cell transplantation patients. J Microbiol Immunol Infect. 2017;50(3):307-313.
doi pubmed - de Pagter PJ, Schuurman R, Visscher H, de Vos M, Bierings M, van Loon AM, Uiterwaal CS, et al. Human herpes virus 6 plasma DNA positivity after hematopoietic stem cell transplantation in children: an important risk factor for clinical outcome. Biol Blood Marrow Transplant. 2008;14(7):831-839.
doi pubmed - Przybylski M, Majewska A, Dzieciatkowski T, Rusicka P, Basak GW, Nasilowska-Adamska B, Bilinski J, et al. Infections due to alphaherpesviruses in early post-transplant period after allogeneic haematopoietic stem cell transplantation: results of a 5-year survey. J Clin Virol. 2017;87:67-72.
doi pubmed - Reed DR, Petroni GR, West M, Jones C, Alfaraj A, Williams PG, DeGregory K, et al. Prophylactic pretransplant ganciclovir to reduce cytomegalovirus infection after hematopoietic stem cell transplantation. Hematol Oncol Stem Cell Ther. 2023;16(1):61-69.
doi pubmed - Chen K, Cheng MP, Hammond SP, Einsele H, Marty FM. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv. 2018;2(16):2159-2175.
doi pubmed pmc - Behrendt CE, Rosenthal J, Bolotin E, Nakamura R, Zaia J, Forman SJ. Donor and recipient CMV serostatus and outcome of pediatric allogeneic HSCT for acute leukemia in the era of CMV-preemptive therapy. Biol Blood Marrow Transplant. 2009;15(1):54-60.
doi pubmed pmc - Matthes-Martin S, Lion T, Aberle SW, Fritsch G, Lawitschka A, Bittner B, Frommlet F, et al. Pre-emptive treatment of CMV DNAemia in paediatric stem cell transplantation: the impact of recipient and donor CMV serostatus on the incidence of CMV disease and CMV-related mortality. Bone Marrow Transplant. 2003;31(9):803-808.
doi pubmed - Ogata M, Oshima K, Ikebe T, Takano K, Kanamori H, Kondo T, Ueda Y, et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(11):1563-1570.
doi pubmed - Silcock R, Mitchell K, Fraser C, Clark J. Epidemiology and outcome for viremia in children undergoing bone marrow transplant: a retrospective cohort study. Transpl Infect Dis. 2021;23(4):e13580.
doi pubmed - Yamada M, Sakamoto K, Tomizawa D, Ishikawa Y, Matsui T, Gocho Y, Sakaguchi H, et al. A prospective viral monitoring study after pediatric allogeneic hematopoietic stem cell transplantation for malignant and nonmalignant diseases. Transplant Cell Ther. 2021;27(10):872.e871-872.e878.
doi pubmed - Wang X, Patel SA, Haddadin M, Cerny J. Post-allogeneic hematopoietic stem cell transplantation viral reactivations and viremias: a focused review on human herpesvirus-6, BK virus and adenovirus. Ther Adv Infect Dis. 2021;8:20499361211018027.
doi pubmed pmc - Atay D, Akcay A, Erbey F, Ozturk G. The impact of alternative donor types on viral infections in pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2018;22(2):e13109.
doi pubmed pmc - Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, Shaw PJ, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority phase III study. J Clin Oncol. 2021;39(4):295-307.
doi pubmed pmc - Yalcin K, Pehlivan B, Celen S, Bas EG, Kabakci C, Pashayev D, Daloglu H, et al. Comparison of total body irradiation-based versus chemotherapy-based conditionings for early complications of allogeneic hematopoietic stem cell transplantation in children with ALL. J Pediatr Hematol Oncol. 2021;43(7):266-270.
doi pubmed - Kullberg-Lindh C, Mellgren K, Friman V, Fasth A, Ascher H, Nilsson S, Lindh M. Opportunistic virus DNA levels after pediatric stem cell transplantation: serostatus matching, anti-thymocyte globulin, and total body irradiation are additive risk factors. Transpl Infect Dis. 2011;13(2):122-130.
doi pubmed - Potdar RR, Gupta S, Giebel S, Savani BN, Varadi G, Nagler A, Blamek S. Current status and perspectives of irradiation-based conditioning regimens for patients with acute leukemia undergoing hematopoietic stem cell transplantation. Clin Hematol Int. 2019;1(1):19-27.
doi pubmed pmc - Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124(3):344-353.
doi pubmed pmc - Nagler A, Mohty M. In 2022, which is preferred: haploidentical or cord transplant? Hematology Am Soc Hematol Educ Program. 2022;2022(1):64-73.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.