Efficacy and Safety of Orelabrutinib for Previously Treated Marginal Zone Lymphoma
DOI:
https://doi.org/10.14740/jh2149Keywords:
Lymphoma, B cell, Marginal zone, Bruton’s tyrosine kinase, Orelabrutinib, SafetyAbstract
Background: This study aimed to investigate the efficacy and safety of orelabrutinib monotherapy in Chinese patients with marginal zone B-cell lymphoma (MZL).
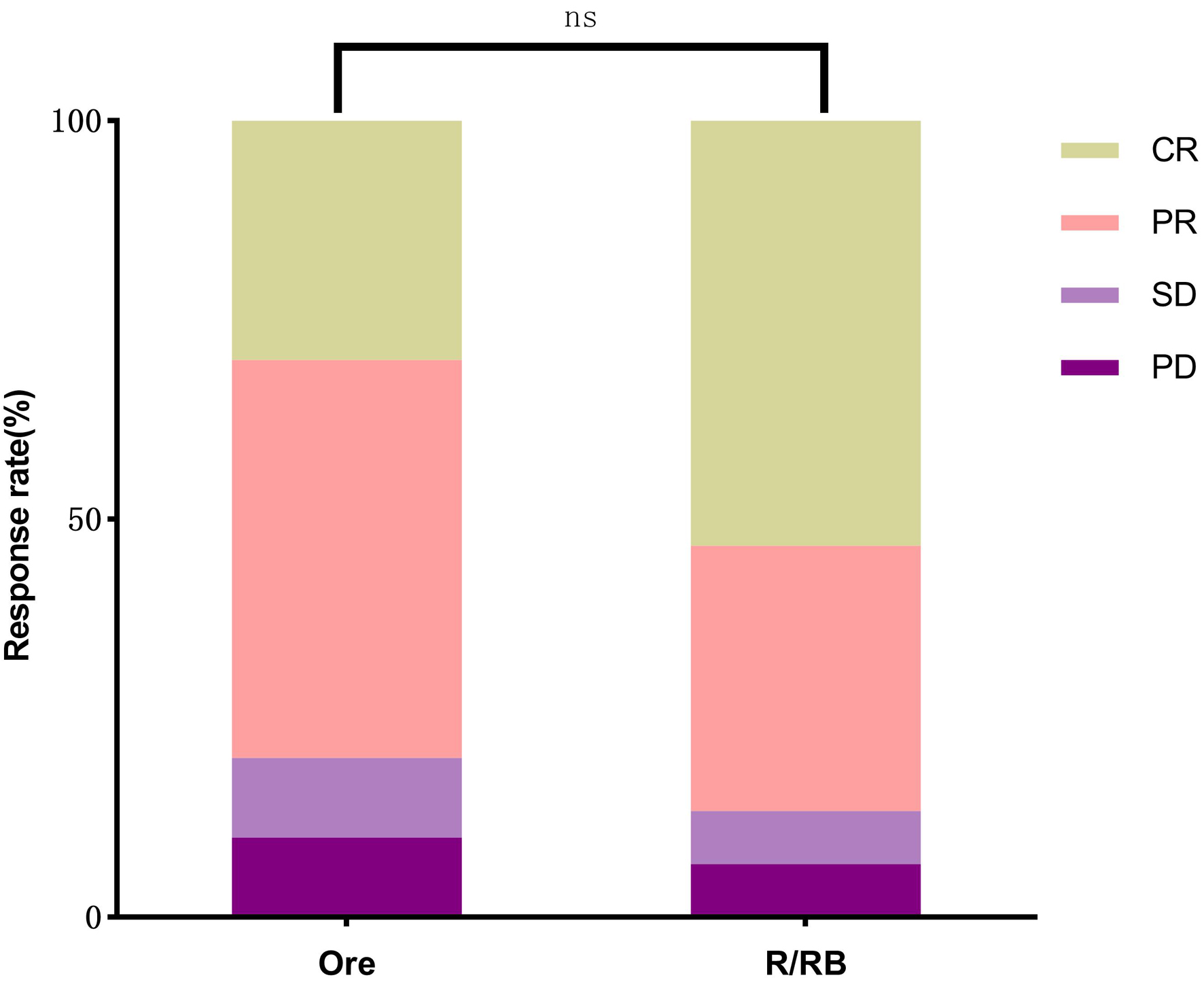
Methods: We conducted a retrospective analysis of MZL patients treated with orelabrutinib monotherapy (n = 30) or rituximab/rituximab plus bendamustine (R/BR) (n = 81) at the Affiliated Hospital of Qingdao University from January 1, 2019, to December 31, 2023. Orelabrutinib monotherapy was assigned to the experimental group. Propensity score matching (PSM) was applied to match 30 MZL patients treated with the R/BR regimen as a control group, comparing the efficacy and safety between the two groups.
Results: In both the orelabrutinib group and the control group, patients who developed relapsed or refractory (R/R) disease after non-rituximab/rituximab-based chemotherapy (non-R/R-CT) therapies (including anti-infective treatment and localized radiotherapy) achieved clinical responses. For 17 patients with R/R disease following R/R-CT treatment, no statistically significant differences were observed between the orelabrutinib group and the BR group in disease control rate (DCR) (82.35% vs. 88.23%, P = 1.00), overall response rate (ORR) (64.70% vs. 76.47%, P = 0.70), and complete response (CR) (17.64% vs. 29.41%, P = 0.68). There was no statistically significant difference in 1-year event-free survival (EFS) (86.66% (95% confidence interval (CI): 68.27 - 94.77) vs. 83.33% (95% CI: 64.49 - 92.70), P = 0.66). Hematologic adverse events (66.66% vs. 86.66%, P = 0.10), lymphopenia (6.66% vs. 40.00%, P < 0.01), and grade 3/4 adverse events (10.00% vs. 33.33%, P = 0.03) were all lower in the orelabrutinib group compared to the control group. Neither group had patients who discontinued treatment due to adverse reactions.
Conclusion: Orelabrutinib monotherapy in MZL possesses favorable efficacy and safety in our study. Given the limited sample size, further clinical research with larger cohorts and extended follow-up is necessary.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








