| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 4, August 2025, pages 223-233
Clinical Patterns and Prognostic Outcomes of Asian Ocular Adnexal Marginal Zone Lymphoma
Rowena Lee Ying Kwana, b, Ryan Mao Heng Limb, Jason Yongsheng Chanb, c, d, e
aYong Loo Lin School of Medicine, National University of Singapore, Singapore 168583, Singapore
bDivision of Medical Oncology, National Cancer Centre Singapore, Singapore, Singapore
cSingHealth Duke-NUS Blood Cancer Centre, Singapore, Singapore
dOncology Academic Clinical Program, Duke-NUS Medical School, Singapore, Singapore
eCorresponding Author: Jason Yongsheng Chan, Division of Medical Oncology, National Cancer Centre Singapore, Singapore 168583, Singapore
Manuscript submitted June 25, 2025, accepted August 4, 2025, published online August 25, 2025
Short title: Outcomes of Ocular Lymphoma in an Asian Population
doi: https://doi.org/10.14740/jh2103
| Abstract | ▴Top |
Background: Ocular adnexal marginal zone lymphoma (OAMZL) is the most common subtype of primary ocular lymphoma and has been rising in incidence in Asian populations.
Methods: We conducted a retrospective review of 95 patients diagnosed with OAMZL within a multi-ethnic cohort from Singapore. Clinical characteristics, survival outcomes including overall survival (OS) and progression-free survival (PFS), and maximum standardized uptake value (SUVmax) on staging F-18 fluorodeoxyglucose positron emission tomography/computed tomography (18-FDG-PET/CT) were investigated.
Results: The cohort comprised 60 males and 35 females, with a median age of 58 years (25 - 88). Median follow-up was 92 months. The most common sites involved were the orbit (49.5%) and lacrimal gland (23.2%). Most patients presented with stage 1 disease (72.6%). Five-year OS and PFS for the whole cohort were 94.9% and 84.1%, respectively. Factors significantly associated with poorer OS included advanced (stage 2-4) disease (hazard ratio (HR) 6.26, 95% confidence interval (CI): 1.69 - 23.19, P = 0.0061), older age above 58 years (HR = 15.29, 95% CI: 4.47 - 52.3, P < 0.0001), and higher mucosa-associated lymphoid tissue International Prognostic Index (MALT-IPI) scores of 2 - 3 compared to low (0) and intermediate (1) scores (HR = 9.28, 95% CI: 1.24 - 69.11, P < 0.0001 and HR = 10.99, 95% CI: 1.34 - 89.94, P < 0.0001), respectively. Older age (HR = 2.41, 95% CI: 1.07 - 5.43, P = 0.0330) and advanced disease (HR = 2.47, 95% CI: 1.07 - 7.03, P = 0.0348) were significantly associated with poorer PFS. Median SUVmax of the lesions was 5.6 (2.1 - 9.6), with significantly higher values in advanced disease.
Conclusions: Our study illustrates the favorable prognosis of OAMZL in an Asian cohort, although particular factors may portend worse survival outcomes.
Keywords: Ocular lymphoma; Rituximab; Prognostication; Positron emission tomography; SUVmax
| Introduction | ▴Top |
Lymphoma of the ocular adnexa is estimated to account for 5-15% of all extranodal non-Hodgkin’s lymphoma and is relatively less studied compared to other lymphoma subtypes. Among primary ocular adnexal lymphomas, ocular adnexal marginal zone lymphoma (OAMZL) is the most common subtype, representing up to 80% of all cases [1, 2]. For reasons unclear, it has been observed that there is a rising trend in the incidence of OAMZL, particularly in Asian countries [3]. In Singapore for example, the incidence of marginal zone lymphoma (MZL) has been increasing from 1998 - 2012, highlighting the growing clinical importance of these lymphomas in the region [4]. The majority of cases arise from extranodal sites, with a peculiar predilection to the orbit, aside to the stomach, lung, skin, thyroid gland, salivary glands [5]. Chronic inflammatory states related to autoimmune disorders or infections have been associated with MZL development by driving lymphomagenesis via chronic antigenic stimulation. Notably, OAMZL has been seen to arise in a background of chronic inflammation related to autoimmune thyroid disease, Sjogren’s syndrome and immunoglobulin G (IgG)4-related disease [2, 6], while Chlamydophila psittaci has been posited to play a role in a subset of cases [7].
In OAMZL, the application of F-18 fluorodeoxyglucose positron emission tomography/computed tomography (18-FDG-PET/CT) in staging assessment remains limited and its clinical utility is controversial. Its sensitivity in detecting OAMZL has been reported to range from 27% to 83.1%, depending on the study [3, 8]. Although maximum standardized uptake values (SUVmax) have been suggested as a potential prognostic factor for OAMZL, research in this area remains limited. Furthermore, there is a notable lack of studies, particularly in Asian populations, which have documented the clinical characteristics and outcomes of patients with OAMZL, highlighting the need for further research in this field.
This study aims to investigate the clinical characteristics and examine survival outcomes in a Southeast Asian patient cohort diagnosed with OAMZL, as well as to explore the relationship of SUVmax values on staging 18-FDG-PET/CT imaging with anatomical site and stage of disease.
| Materials and Methods | ▴Top |
Study cohort
Patients diagnosed with OAMZL and seen at the National Cancer Centre Singapore between 1998 and 2024 were retrospectively reviewed. Patients with MZL involving the ocular adnexa, with or without metastatic spread, were included. Pathological diagnosis was confirmed by expert hematopathologists, and all tumor cells were confirmed to express B-cell specific antigens including CD20. A total of 95 patients were included in the final analysis. All data were obtained at the time of diagnosis or subsequent follow-up. The research study was carried out with approval from the SingHealth Centralized Institutional Review Board. Informed consent for the use of biospecimens was obtained in accordance with the Declaration of Helsinki.
Demographic and clinicopathological analysis
Demographical information available included age, sex and ethnicity. Clinical characteristics of each patient such as presenting symptoms, sites of disease involvement, serum lactate dehydrogenase (LDH) level, SUVmax values on FDG-PET/CT imaging, treatment regimens and response to treatment were included in the study. The mucosa-associated lymphoid tissue International Prognostic Index (MALT-IPI) score was calculated based on based on three key clinical parameters (1 point each): age ≥ 70 years, Ann Arbor stage III or IV, and elevated LDH levels [9].
Study endpoints
The outcomes of interest in this study were progression-free survival (PFS) and overall survival (OS). PFS was defined as the time elapsed between the date of diagnosis till the date of relapse, progression, or death from any cause. OS was calculated from the date of diagnosis up till the date of death from any cause or was censored at the date of last follow-up for survivors.
Statistical analysis
Statistical analysis was carried out using methods as previously described [10]. Continuous variables were compared using the Mann-Whitney U test or Kruskal-Wallis test. For each individual clinicopathological parameter, Kaplan-Meier curves were plotted to estimate survival. The log-rank test was then used to calculate hazard ratio (HR), the corresponding 95% confidence interval (CI) and the P values. Clinicopathological parameters found to be significant on univariate analysis using a two-sided test with significance level of 0.05 were identified. All tests were performed using MedCalc statistical software for Windows version 19.0.4 (MedCalc Software, Ostend, Belgium).
| Results | ▴Top |
Patient demographics and clinicopathological characteristics
A total of 95 patients were included in this study. The median age of diagnosis was 58 years (range: 25 to 88 years). Sixty (63.2%) were male and 35 (36.8%) were female. The ethnicity groups of our patient population included Chinese (n = 76, 80.0%), Malay (n = 10, 10.5%) and others (n = 9, 9.5%). Most patients presented with stage 1 disease (n = 69, 72.6%), while the others presented with stage 2 (n = 8, 8.4%), stage 3 (n = 2, 2.1%) and stage 4 disease (n = 16, 16.8%). The lesions were mostly unilateral (n = 76, 80.0%). Bilateral lesions confined to the ocular adnexa were classified as stage 1 disease [11]. The most frequent presenting symptoms were swelling (n = 31, 32.6%), presence of a lump (n = 20, 21.1%), and proptosis (n = 18, 18.9%). The most common site of disease involvement was the orbit (n = 47, 49.5%) which consists of the extraocular muscles, blood vessels, nerves and connective tissue, followed by the lacrimal gland (n = 22, 23.2%), conjunctiva (n = 15, 15.8%), and eyelid (n = 11, 11.6%). Autoimmune diseases were concurrently diagnosed in 12 patients, including IgG4 disease (n = 9), Sjogren’s syndrome (n = 2), and an unspecified connective tissue disease (n = 1). The most common nodal sites of disease spread at diagnosis were lymph nodes located above the diaphragm (n = 14, 14.7%). The most common extranodal sites of disease spread at diagnosis were the lung, paravertebral space, renal pelvis and stomach (n = 3 each). Serum LDH level was elevated in 27 patients (28.4%). MALT-IPI scores were low (0) for 39 patients (41.1%), intermediate (1) for 38 patients (40.0%), and high (2 - 3) for 10 patients (10.5%). This information is summarized in Table 1.
 Click to view | Table 1. Demographic and Clinical Characteristics of Patients With OAMZL |
Treatment outcomes and survival analyses
The median follow-up duration for all patients was 92 months. The 5-year OS and PFS rates were 94.9% and 84.1%, respectively. Median OS was not reached, while the median PFS was 194 months (Fig. 1). For stage 1 disease, the majority of patients received orbital radiotherapy alone as initial treatment (n = 61 of 69, 88.4%). Only two patients received systemic therapy (rituximab alone and rituximab plus bendamustine, n = 1 each). The rest underwent excision alone (n = 2) or were managed by watchful waiting (n = 4). For stage 2-4 disease, 11 of 26 (42.3%) patients received radiotherapy alone. Four patients were managed by watchful waiting (n = 4), while the rest received various systemic therapies including rituximab alone (n = 3), R-CVP (rituximab, cyclophosphamide, vincristine, prednisone) (n = 4), and other chemotherapies (n = 4) (Table 2). Subsequent lines of treatment for patients with progression or relapse of disease included radiotherapy as the most common modality (n = 5 and n = 4 for stage 1 and stage 2-4 disease, respectively), followed by rituximab (n = 4 and n = 2, respectively) (Fig. 2).
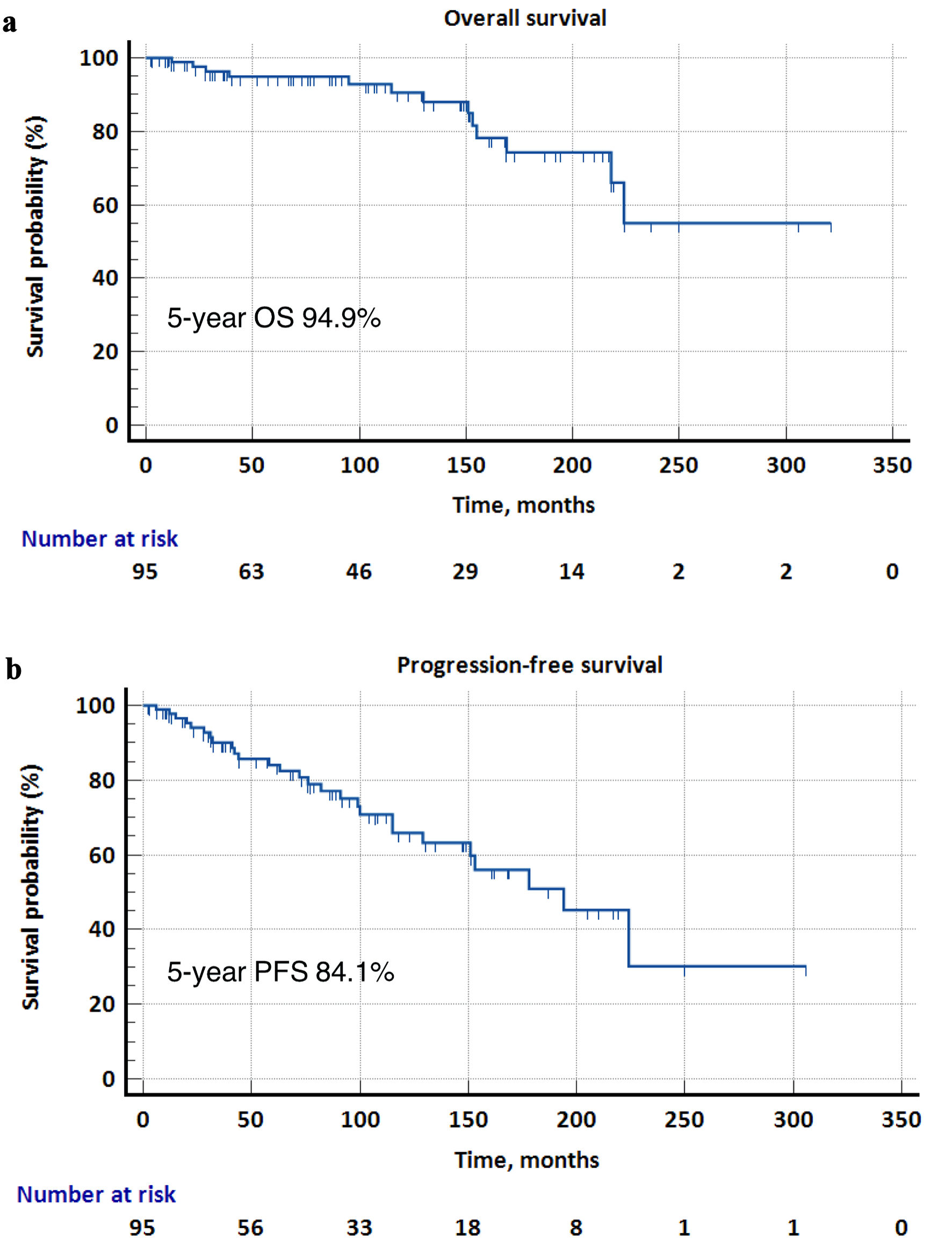 Click for large image | Figure 1. Kaplan-Meier survival curves for overall survival (OS) and progression-free survival (PFS) in the overall cohort. (a) The 5-year OS was 94.9%, and the median OS was not reached. (b) The 5-year PFS was 84.1%, and the median PFS was 194 months. |
 Click to view | Table 2. First-Line Management of Patients With Ocular Lymphoma in the Study Cohort |
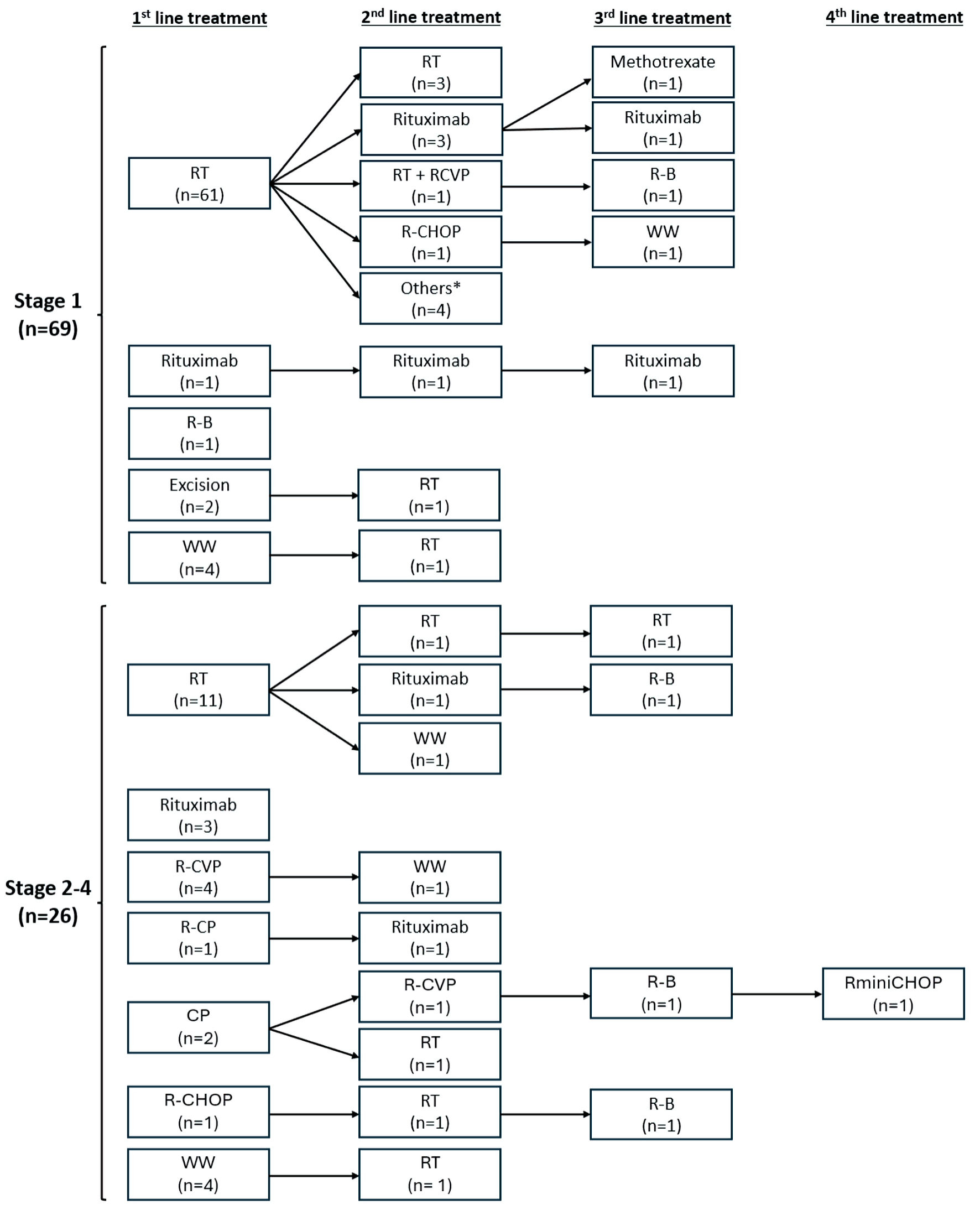 Click for large image | Figure 2. First- and subsequent-line treatment therapies stratified by disease stage. Others: R-B (n = 2), bendamustine alone (n = 1), CVP (n = 1). CP: chlorambucil, prednisone; R-CP: rituximab, chlorambucil, prednisone; CVP: cyclophosphamide, vincristine, prednisone; R-CVP: rituximab, cyclophosphamide, vincristine, prednisone; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; RminiCHOP: rituximab plus reduced dose CHOP; R-B: rituximab, bendamustine; RT: radiation therapy; WW: watchful waiting. |
Prognostic factors for OS
In our cohort, a total of 12 patients had died as of last follow-up. The causes of death included other secondary cancers (n = 4), infection (n = 3), MZL (n = 2), end-stage renal failure (n = 1), and unknown (n = 2). In univariate analyses, patients with stage 2-4 disease had significantly poorer OS than patients with stage 1 disease (HR = 6.26, 95% CI: 1.69 - 23.19, P = 0.0061). Median survival for stage 1 was not reached, while for stage 2-4 it was 169 months. 5-year survival was 96.6% in stage 1 and 90.5% in stage 2-4. Patients who were at the median age of 58 and older at diagnosis had significantly poorer OS than patients aged below 58 years (HR = 15.29, 95% CI: 4.47 - 52.3, P < 0.0001). Median survival was 155 months for patients aged 58 and above and not reached for the younger group. The 5-year OS rate was 89.4% in older patients and 100% in younger patients (Fig. 3a, b).
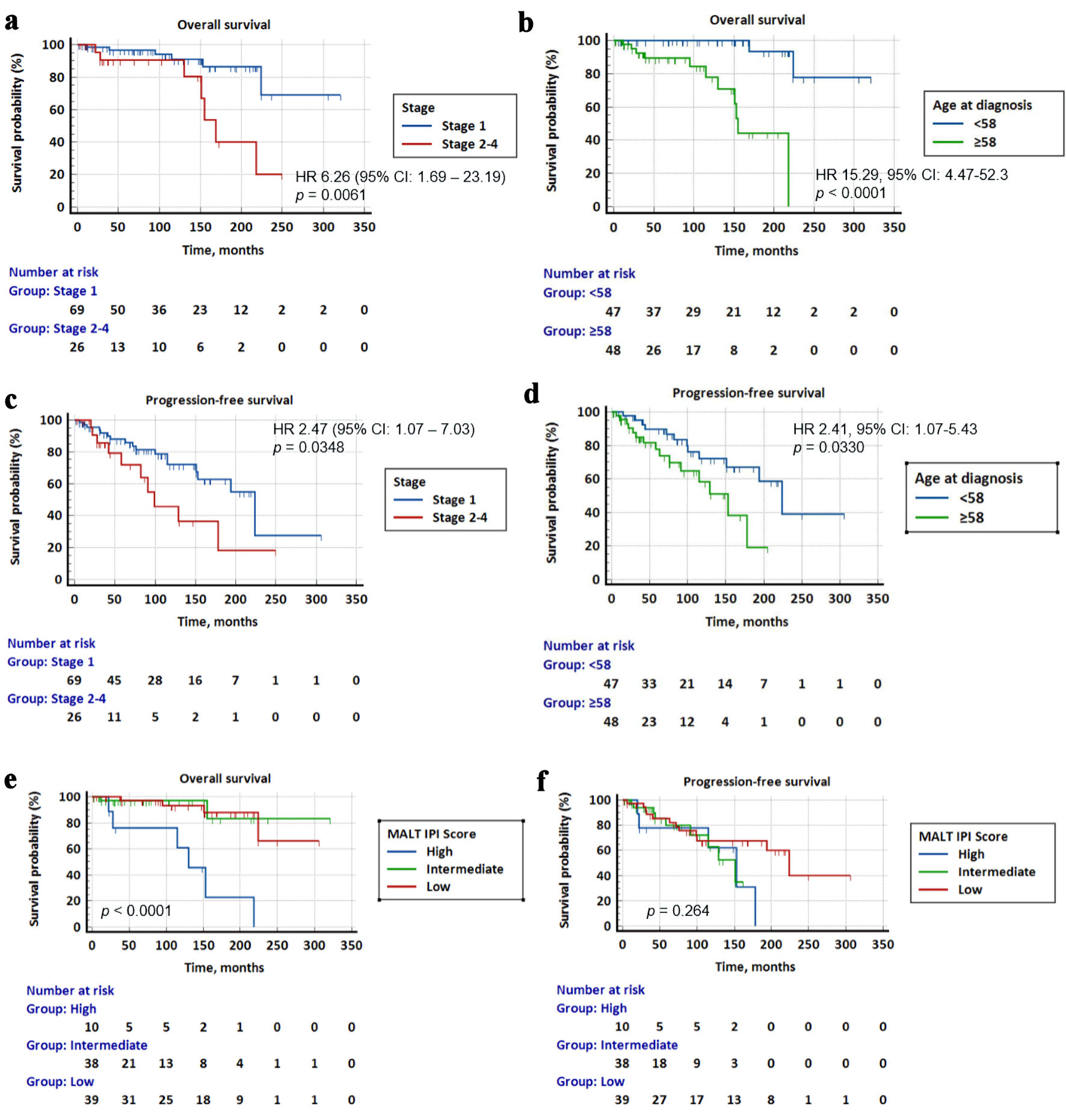 Click for large image | Figure 3. Kaplan-Meier survival curves for overall survival (OS) and progression-free survival (PFS) stratified by Ann Arbor stage, age at diagnosis and MALT-IPI score. (a) Patients with stage 2-4 disease had significantly poorer survival than patients with stage 1 disease (HR = 6.26, 95% CI: 1.69 - 23.19, P = 0.0061). (b) Patients above 58 years had significantly lower survival than younger patients (HR = 15.29, 95% CI: 4.47 - 52.3, P < 0.0001). (c) Patients with stage 2-4 disease had a significantly poorer PFS than patients with stage 1 disease (HR = 2.47, 95% CI: 1.07 - 7.03, P = 0.0348). (d) Patients above 58 years had a significantly lower PFS than younger patients (HR = 2.41, 95% CI: 1.07 - 5.43, P = 0.0330). (e) OS for low vs. intermediate vs. high MALT-IPI score. Patients with high MALT-IPI score had significantly worse OS than patients with low and intermediate IPI scores (HR = 9.28, 95% CI: 1.24 - 69.11, P < 0.0001, and HR = 10.99, 95% CI: 1.34 - 89.94, P < 0.0001) respectively. (f) PFS for low vs. intermediate vs. high MALT-IPI score. There was no significant difference in PFS of patients with different MALT-IPI scores. MALT-IPI: mucosa-associated lymphoid tissue International Prognostic Index; HR: hazard ratio; CI: confidence interval. |
Patients with stage 2-4 disease had significantly poorer PFS than patients with stage 1 disease (HR = 2.47, 95% CI: 1.07 - 7.03, P = 0.0348). Median PFS was 224 months for stage 1 disease, and 99 months for stage 2-4. The 5-year PFS was 88.0% for stage 1 and 71.9% for stage 2-4. Patients who were at the median age of 58 and above at diagnosis had significantly lower PFS than those aged below 58 (HR = 2.41, 95% CI: 1.07 - 5.43, P = 0.0330). Median PFS was 153 months in the older group compared to 224 months in the younger group. The 5-year PFS was 77.7% in older patients and 89.7% in younger patients (Fig. 3c, d). There was no significant difference in OS or PFS when comparing patients with unilateral and bilateral disease.
MALT-IPI risk groups and survival outcomes
In terms of MALT-IPI score, patients with high scores (2 - 3) had significantly worse OS than patients with low (0) and intermediate (1) scores (HR = 9.28, 95% CI: 1.24 - 69.11, P < 0.0001 and HR = 10.99, 95% CI: 1.34 - 89.94, P < 0.0001), respectively. Median survival was 130 months for patients with high MALT-IPI scores and not reached for those with low and intermediate scores. The 5-year OS was 97.0% for patients with low and intermediate MALT-IPI scores, and 76.2% for patients with high scores (Fig. 3e). There was no significant difference in PFS of patients with low (0), intermediate (1) and high (2 - 3) scores (Fig. 3f).
SUVmax values on 18-FDG PET/CT and clinical characteristics
In an exploratory analysis, 18-FDG PET/CT images of OAMZL at diagnosis were retrieved, and SUVmax values were collated. Representative images of selected cases based on anatomical location are shown (Fig. 4a-d). The median level of SUVmax of the lesions was 5.6 (range: 2.1 - 9.6). We observed that patients with stage 2-4 disease (median: 7.8, range: 2.1 - 9.6) had significantly higher SUVmax values compared to patients with stage 1 disease (median: 4.4, range: 2.4 - 6.8) (P = 0.035). Interestingly, although there was no significant difference between SUVmax values across different tumor sites, eyelid tumors had numerically higher uptake values (P = 0.18, Kruskal-Wallis test) (Fig. 4e, f). The median SUVmax values for eyelid, lacrimal gland, orbit and conjunctival tumors were 7.8, 6.1, 5.5 and 4.0, respectively. An overview of studies on 18FDG-PET/CT in OAMZL, from 2010 to 2025, is included here (Supplementary Material 1, jh.elmerpub.com).
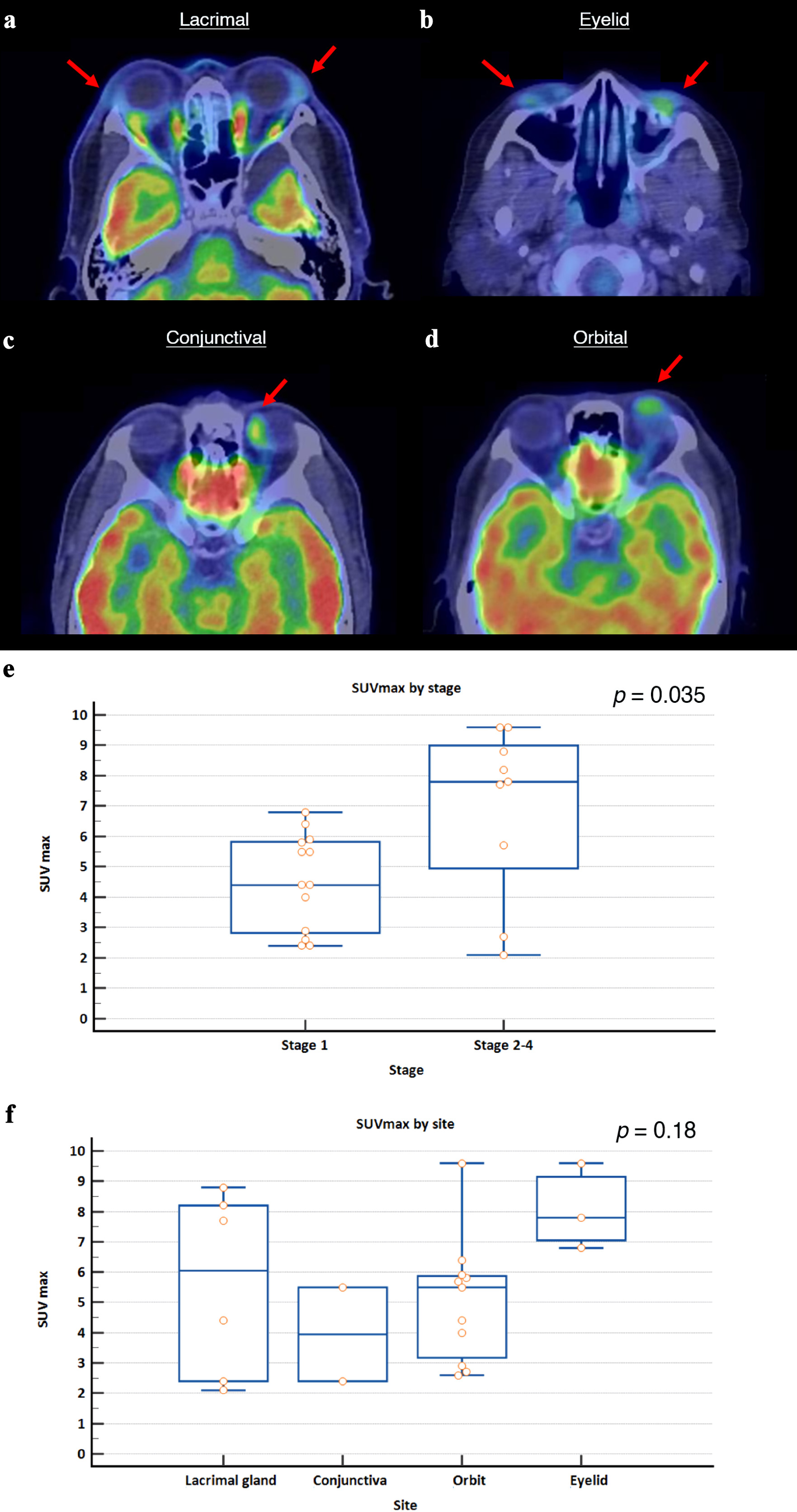 Click for large image | Figure 4. Representative 18-FDG PET/CT images of ocular marginal zone lymphoma based on anatomical location, and SUVmax values at diagnosis stratified by Ann Arbor stage and tumor site. (a) Bilateral enlarged and FDG-avid lacrimal glands (left, SUVmax 3.1; right, SUVmax 8.8). (b) Bilateral FDG-avid lower eyelid masses (left, SUVmax 7.8; right, SUVmax 4.0). (c) FDG-avid mass over medial-superior aspect of the left globe (SUVmax 5.5). (d) FDG-avid nodular lesion on the left orbit (SUVmax 4.4). Red arrows indicate the site of disease. (e) Patients with stage 2-4 disease had significantly higher SUVmax values compared to patients with stage 1 disease (P = 0.035, Mann-Whitney U test). (f) There was no significant difference between SUVmax values across different tumor sites, though eyelid tumors had numerically higher uptake values (P = 0.18, Kruskal-Wallis test). SUVmax: maximum standardized uptake value. |
| Discussion | ▴Top |
This study investigates the outcomes of ocular lymphoma in a Southeast Asian population in Singapore. The median age of presentation in our cohort was 58 years, which is similar to Chinese patients (median: 57, n = 166), but younger than the median age of 66 in American patients (n = 23) and 62 years in an international cohort (n = 689) [12-14]. The 5-year OS and PFS for stage 1 OAMZL was 96.6% and 88.0%, respectively, in contrast to the Chinese study where OS and PFS was 99% and 76%, respectively for stage 1 disease [12]. The 5-year OS and PFS for the entire cohort was 94.9% and 84.1%, respectively, similar to 96% and 79% in an American population (n = 87), and higher than the OS of 83% reported in an international population [14, 15]. These findings suggest that the earlier age of presentation in Asian populations may not have negative implications on OS and PFS.
The most common sites of disease involvement in our cohort were the orbit (49.5%) and lacrimal gland (23.2%). Other larger studies in predominantly Caucasian populations have reported that ocular lymphomas primarily present in the orbit followed by conjunctiva, with the lacrimal gland being a less common site of involvement [16, 17]. Similarly, a Taiwanese study (n = 112) identified the orbit, followed by conjunctiva and lacrimal gland (35.5%, 31.6% and 27.6%, respectively) as the three most common sites of disease [18]. Interestingly, our findings align with a Thai study (n = 121), which reported the orbit, followed by lacrimal apparatus, being the most common sites of disease (46.3% and 34.7%, respectively) [19]. This observation could potentially suggest different patterns of disease involvement in Southeast Asian populations.
The most common sites of disease relapse after treatment were the lacrimal gland and lung (n = 5 each), followed by cervical lymph nodes and the orbit (n = 4 each). This is in line with findings from a Danish study, which reported the most common sites of relapse to be the eye, followed by lymph nodes and the lung [20].
The MALT-IPI divides patients into low, intermediate, and high-risk groups [9]. This index has been shown to be a valuable tool in predicting the prognoses of MALT lymphoma patients [21]. In our study, patients with higher IPI scores had significantly lower OS but not PFS. This is in contrast to a Korean study that showed significantly lower PFS but not OS for patients with higher MALT-IPI scores [22]. This difference could possibly be attributed to several factors. Our study had a substantially greater proportion of patients who exhibited high-risk features, including both elevated serum LDH (28.4% versus 7.2% in the Korean cohort) and Ann Arbor stage III/IV disease (18.9% versus 10.6% in Korea), resulting in fewer IPI low-risk (41.1% versus 82.6%) and more intermediate- to high-risk (10.5% versus 6.3%) classifications in our cohort. A high IPI score reflects advanced age, higher LDH levels and disease stage - factors related to aggressiveness and severity of the lymphoma resulting in shorter OS. PFS, on the other hand, can be influenced by other factors such as frequency of assessment and treatment response depending on therapeutic strategy employed [23].
A total of 22 patients underwent PET/CT scans at the point of staging, and SUVmax values of their ocular tumors were recorded. In patients with bilateral disease, the higher value was taken into account. Patients with stage 2-4 disease had significantly higher SUVmax than those with stage 1 disease. Eyelid tumors had the highest mean SUVmax (8.1 ± 1.2), although this was not statistically significant. This differs from a Korean study, which showed orbit and lacrimal gland tumors having the highest and lowest mean SUVmax values, respectively [3]. It has been found that SUVmax values of the single most metabolically active lesion can be useful in response evaluation and prognosis prediction in indolent lymphomas [24]. However, in our study, survival analysis was unable to be performed based on SUVmax values due to insufficient data.
Our exploratory analysis of OAMZL patients who underwent PET/CT scans during staging showed that patients with advanced disease had significantly higher PET/CT SUVmax scores of their primary ocular tumor, as compared to those with localized disease. Although no study has reviewed the correlation between SUVmax score and prognostic stage in OAZML, other studies involving solid tumors such as lung adenocarcinomas [25] and breast [26] cancers have found that higher SUVmax scores correlated with increased prognostic stage in these cancers. Conversely, while our study found that OAMZL tumors arising from the eyelid had non-significantly higher mean SUVmax values as compared to other orbital sites, both Park et al [3] and Wang et al [27] reported that SUVmax of orbital masses were significantly elevated as compared to other sites such as conjunctiva, lacrimal gland and eyelid. This could suggest that the relationship between SUVmax values and tumor sites is less apparent, or that these results may be non-conclusive due to the relatively small sample sizes of each study.
Other recent studies investigating the utility of SUVmax values in assessing treatment response and prognosis showed that OAMZL tumors had a decrease in SUVmax values after effective treatment [5], and that PET/CT FDG-avid OAMZL tumors are more likely to achieve complete remission as compared to non-FDG-avid tumors [27]. As our study was unable to perform survival analysis with regard to PET/CT FDG-avidity and SUVmax values for FDG-avid tumors due to insufficient data, further follow-up studies should be performed to validate these findings so as to improve prognostication at diagnosis and across treatment for OAMZL patients. Finally, as some studies have shown that CT and/or MRI scans have a higher sensitivity of detecting ocular adnexal lymphoproliferative disease as compared to PET/CT scan [28, 29], the role of PET/CT scan may be best suited for prognostication of survival and treatment response, as well as in further staging [3], as compared to making a primary diagnosis of OAMZL.
Our study has certain limitations inherent to its retrospective nature, including gaps in data collection. Additionally, due to the small number of patients who underwent PET/CT scans and subsequently progressed or relapsed, survival analysis based on SUVmax values could not be performed. Furthermore, information on subsequent relapse and treatment may be unavailable for patients who lost to follow-up.
In conclusion, our study illustrates the favorable survival outcomes of OAMZL and demonstrates certain similarities in the presentation of OAMZL among Asian populations, such as age of presentation and site of disease involvement, when compared to Western populations.
| Supplementary Material | ▴Top |
Suppl 1. Overview of studies on 18FDG-PET/CT in OAMZL, from 2010 to 2025.
Acknowledgments
The authors would like to thank all participants in the study.
Financial Disclosure
This work was supported by the Singapore Ministry of Health’s National Medical Research Council Research Transition Award (TA21jun-0005), Large Collaborative Grant (OFLCG-23May0039), and TETRAD II Collaborative Centre Grant (CG21APR2002), SingHealth Duke-NUS AM/ACP-Designated Philanthropic Fund Grant Award (08/FY2023/EX/27-A65), as well as the Khoo Bridge Funding Award (Duke-NUS-KBrFA/2025/0090) provided by Duke-NUS Medical School and the “Estate of Tan Sri Khoo Teck Puat”.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Participants and/or their legal guardians provided informed consent for their data to be used in this research.
Author Contributions
RLK, RML and JYC analyzed the data and drafted the manuscript; RLK and JYC obtained patient data; JYC designed the study, interpreted the results, and revised the manuscript. All authors read and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
OAMZL: ocular adnexal marginal zone lymphoma; 18-FDG-PET/CT: F-18 fluorodeoxyglucose positron emission tomography/computed tomography; MZL: marginal zone lymphoma; LDH: lactate dehydrogenase; PFS: progression-free survival; OS: overall survival; R-CVP: rituximab, cyclophosphamide, vincristine, prednisolone; CP: chlorambucil, prednisone; R-CP: rituximab, chlorambucil, prednisone; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-B: rituximab, bendamustine
| References | ▴Top |
- Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66(2):153-171.
doi pubmed - Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114(3):501-510.
doi pubmed - Park HL, O JH, Park SY, Jung SE, Park G, Choi BO, Kim SH, et al. Role of F-18 FDG PET/CT in non-conjunctival origin ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphomas. EJNMMI Res. 2019;9(1):99.
doi pubmed - Lim RB, Loy EY, Lim GH, Zheng H, Chow KY, Lim ST. Gender and ethnic differences in incidence and survival of lymphoid neoplasm subtypes in an Asian population: Secular trends of a population-based cancer registry from 1998 to 2012. Int J Cancer. 2015;137(11):2674-2687.
doi pubmed - Teo YH, Teo YN, Khoo LP, Chang EWY, Tan YH, Chiang J, Yang VS, et al. Clinicopathological factors affecting prognosis in marginal zone lymphoma in Asian patients: a cohort study. Leuk Lymphoma. 2022;63(11):2723-2726.
doi pubmed - Ohno K, Sato Y, Ohshima K, Takata K, Miyata-Takata T, Takeuchi M, Gion Y, et al. A subset of ocular adnexal marginal zone lymphomas may arise in association with IgG4-related disease. Sci Rep. 2015;5:13539.
doi pubmed - Collina F, De Chiara A, De Renzo A, De Rosa G, Botti G, Franco R. Chlamydia psittaci in ocular adnexa MALT lymphoma: a possible role in lymphomagenesis and a different geographical distribution. Infect Agent Cancer. 2012;7:8.
doi pubmed - Valenzuela AA, Allen C, Grimes D, Wong D, Sullivan TJ. Positron emission tomography in the detection and staging of ocular adnexal lymphoproliferative disease. Ophthalmology. 2006;113(12):2331-2337.
doi pubmed - Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, Martelli M, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409-1417.
doi pubmed - Tan KM, Chia B, Lim JQ, Khoo LP, Cheng CL, Tan L, Poon E, et al. A clinicohaematological prognostic model for nasal-type natural killer/T-cell lymphoma: A multicenter study. Sci Rep. 2019;9(1):14961.
doi pubmed - Lee SE, Paik JS, Cho WK, Choi BO, Lee SN, Jung SE, Park KS, et al. Feasibility of the TNM-based staging system of ocular adnexal extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). Am J Hematol. 2011;86(3):262-266.
doi pubmed - Liang Y, Fu RY, Liu XL, Liu XD, Piao YS, Ma JM, Wang L. Long-term survival outcomes of patients with primary ocular adnexal MALT lymphoma: A large single-center cohort study. Cancer Med. 2023;12(3):2514-2523.
doi pubmed - Charlotte F, Doghmi K, Cassoux N, Ye H, Du MQ, Kujas M, Lesot A, et al. Ocular adnexal marginal zone B cell lymphoma: a clinical and pathologic study of 23 cases. Virchows Arch. 2006;448(4):506-516.
doi pubmed - Hindso TG, Esmaeli B, Holm F, Mikkelsen LH, Rasmussen PK, Coupland SE, Finger PT, et al. International multicentre retrospective cohort study of ocular adnexal marginal zone B-cell lymphoma. Br J Ophthalmol. 2020;104(3):357-362.
doi pubmed - Rosado MF, Byrne GE, Jr., Ding F, Fields KA, Ruiz P, Dubovy SR, Walker GR, et al. Ocular adnexal lymphoma: a clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood. 2006;107(2):467-472.
doi pubmed - Moslehi R, Devesa SS, Schairer C, Fraumeni JF, Jr. Rapidly increasing incidence of ocular non-hodgkin lymphoma. J Natl Cancer Inst. 2006;98(13):936-939.
doi pubmed - Desai A, Joag MG, Lekakis L, Chapman JR, Vega F, Tibshirani R, Tse D, et al. Long-term course of patients with primary ocular adnexal MALT lymphoma: a large single-institution cohort study. Blood. 2017;129(3):324-332.
doi pubmed - Hsu CR, Chen YY, Yao M, Wei YH, Hsieh YT, Liao SL. Orbital and ocular adnexal lymphoma: a review of epidemiology and prognostic factors in Taiwan. Eye (Lond). 2021;35(7):1946-1953.
doi pubmed - Seresirikachorn K, Norasetthada L, Ausayakhun S, Apivatthakakul A, Tangchittam S, Pruksakorn V, Wudhikarn K, et al. Clinical presentation and treatment outcomes of primary ocular adnexal MALT lymphoma in Thailand. Blood Res. 2018;53(4):307-313.
doi pubmed - Sjo LD, Heegaard S, Prause JU, Petersen BL, Pedersen S, Ralfkiaer E. Extranodal marginal zone lymphoma in the ocular region: clinical, immunophenotypical, and cytogenetical characteristics. Invest Ophthalmol Vis Sci. 2009;50(2):516-522.
doi pubmed - Hong J, Cho J, Ko YH, Kim SJ, Kim WS. Validation of the marginal zone lymphoma international prognostic index. Ann Hematol. 2019;98(2):457-464.
doi pubmed - Jeon YW, Yang HJ, Choi BO, Jung SE, Park KS, O JH, Yang SW, et al. Comparison of selection and long-term clinical outcomes between chemotherapy and radiotherapy as primary therapeutic modality for ocular adnexal MALT lymphoma. EClinicalMedicine. 2018;4-5:32-42.
doi pubmed - Haslam A, Gill J, Prasad V. The frequency of assessment of progression in randomized oncology clinical trials. Cancer Rep (Hoboken). 2022;5(7):e1527.
doi pubmed - Kim HJ, Lee R, Choi H, Paeng JC, Cheon GJ, Lee DS, Chung JK, et al. Application of quantitative indexes of FDG PET to treatment response evaluation in indolent lymphoma. Nucl Med Mol Imaging. 2018;52(5):342-349.
doi pubmed - Sun X, Chen T, Xie C, Liu L, Lei B, Wang L, Ruan M, et al. Relationships between SUVmax of lung adenocarcinoma and different T stages, histological grades and pathological subtypes: a retrospective cohort study in China. BMJ Open. 2022;12(5):e056804.
doi pubmed - Mori M, Fujioka T, Kubota K, Katsuta L, Yashima Y, Nomura K, Yamaga E, et al. Relationship between prognostic stage in breast cancer and fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. J Clin Med. 2021;10(14):3173.
doi pubmed - Wang W, Ni X, Tang T, Wang J, Li Y, Song X. The role of (18)F-FDG PET/CT in diagnosis and treatment evaluation for ocular adnexal mucosa-associated lymphoid tissue lymphoma. Br J Radiol. 2022;95(1130):20210635.
doi pubmed - Zanni M, Moulin-Romsee G, Servois V, Validire P, Benamor M, Plancher C, Rouic LL, et al. Value of 18FDG PET scan in staging of ocular adnexal lymphomas: a large single-center experience. Hematology. 2012;17(2):76-84.
doi pubmed - English JF, Sullivan TJ. The role of FDG-PET in the diagnosis and staging of ocular adnexal lymphoproliferative disease. Orbit. 2015;34(5):284-291.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.