Efficacy of Short-Course High-Dose Oral Prednisolone in Rapid Platelet Recovery for Pediatric Acute Immune Thrombocytopenic Purpura: A Prospective Cohort Study
DOI:
https://doi.org/10.14740/jh2052Keywords:
Immune thrombocytopenic purpura, Thrombocytopenia, Corticosteroids, PrednisoloneAbstract
Background: Standard management of acute immune thrombocytopenic purpura (ITP) remains debated, with some advocating observation for mild cases, while others recommend pharmacological intervention to accelerate platelet recovery in children with severe thrombocytopenia (TCP) (platelet count < 20,000/mm3) or significant mucosal bleeds. Corticosteroids, particularly prednisolone (PDN), are a widely used first-line treatment due to their rapid immunosuppressive effect. This prospective cohort study evaluated the effectiveness of short-course high-dose PDN (4 mg/kg/day for 7 days) in treating children aged 1 - 12 years with newly diagnosed acute ITP. The study aimed to assess the clinical and hematological profile of these children and the mean time to platelet recovery.
Methods: A total of 61 patients were enrolled, and their response to treatment was monitored at various intervals, including 48 h, 72 h, day 7, 1 month, and 3 months.
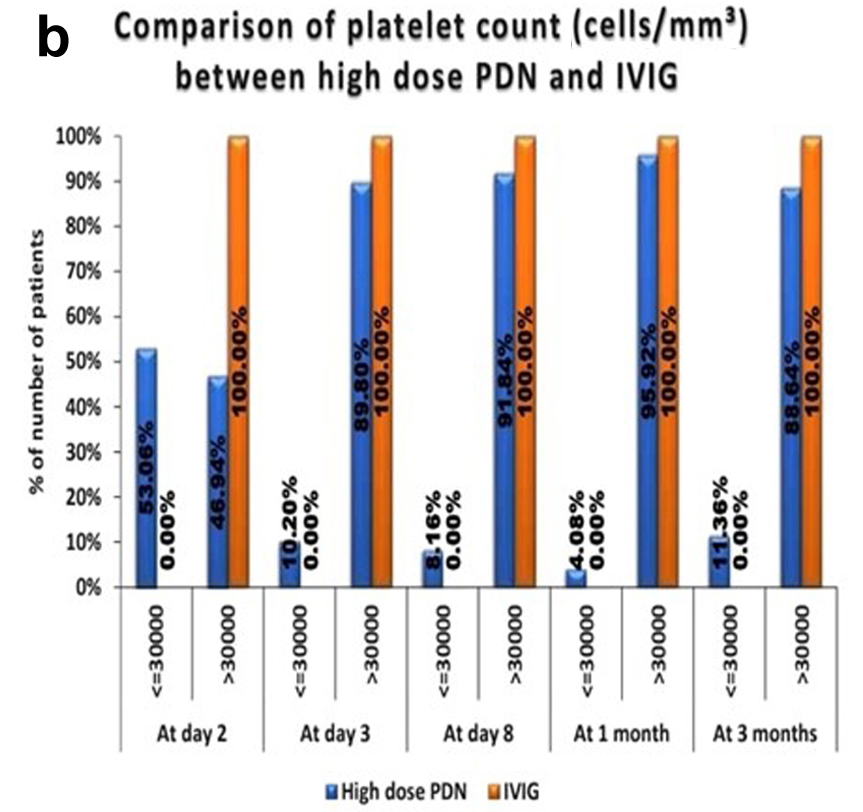
Results: Results revealed a rapid platelet recovery in patients receiving high-dose PDN, with 83.6% of patients achieving platelet counts greater than 50,000/mm3 by day 7. Additionally, 91.8% maintained platelet recovery at 1 month. The study also found that the age group 6 - 12 years had a higher risk of persistent ITP (24.5%), highlighting the importance of close monitoring in this demographic. While the treatment was generally well tolerated, some mild side effects like hypertension and glycosuria were observed.
Conclusion: The study suggests that short-course high-dose PDN can be an effective first-line therapy for acute ITP. It promotes faster platelet recovery and reduces hospitalization time with minimal adverse effects.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








