Successful Second Hematopoietic Stem Cell Transplantation Using Total Body Irradiation-Based Conditioning for Children With Transfusion-Dependent Beta-Thalassemia
DOI:
https://doi.org/10.14740/jh1378Keywords:
Stem cell transplantation, Pediatric, Thalassemia, Second transplant, Graft failure, Survival benefitsAbstract
Background: Graft rejection (GR) occurs in a significant proportion of individuals with transfusion-dependent β-thalassemia (TDT) following hematopoietic stem cell transplantation (HSCT). There have been limited data on the outcome and complications of second HSCT in β-thalassemia patients. The objective was to assess the survival benefits and outcome of second allogeneic HSCT in pediatric TDT patients using Cytoxan (CY) and total body irradiation (TBI) regimen.
Methods: This was a retrospective study on the analysis of the data for 15 patients who had graft failure over an 18-year period (March 2000 to March 2017) at our institution. For the first failed transplants for patients who had a myeloablative regimen consisting of busulfan (BU)-CY with or without additional anti-thymocyte globulin (ATG), the median age at transplant was 4.2 years. Graft failure occurred over a median of 8.6 months (range, 0.6 - 74.3 months) after the first transplant. The median time to the second transplant from GR was 25.3 months. For the second transplant, the same human leukocyte antigen (HLA) match-related donors for the first HSCT were used. Over half of the patients had moderate to severe iron overload with pre-transplant serum ferritin of 1,405 to 4,051 µg/L at transplant.
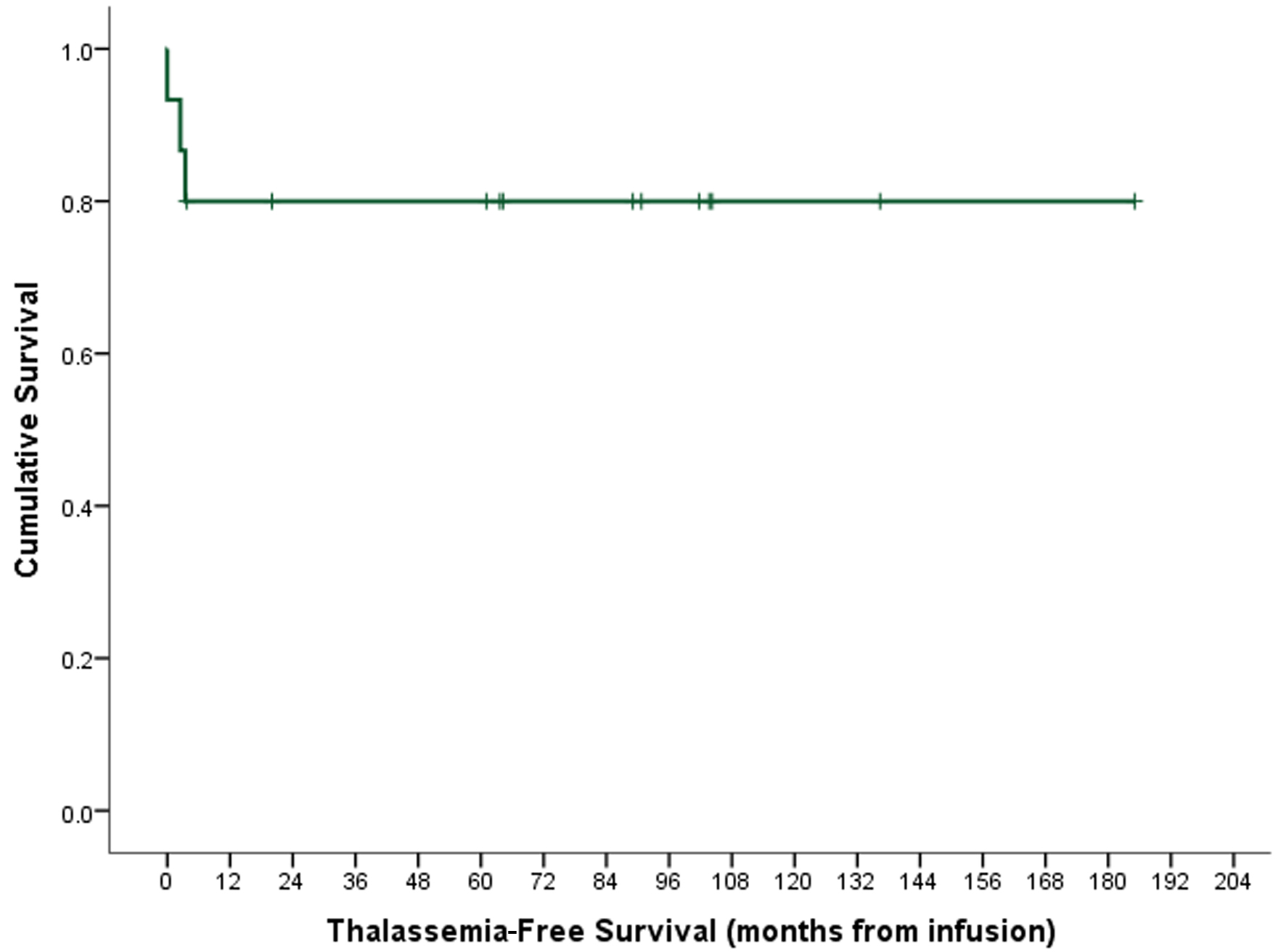
Results: Thirteen patients (86.7%) engrafted with thalassemia-free survival (TFS) of 80.0%. One patient rejected the graft and died. Another died due to infectious complications. Apart from a mild chronic graft-versus-host disease (GvHD) in one patient, no serious complications were observed.
Conclusion: CY-TBI can be used as conditioning for second HSCT in patients with TDT GR following myeloablative conditioning. We observed overall survival and TFS of 87% and 80% respectively with low rejection rate and mortality, and limited long-term side effects.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








