| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 6, December 2025, pages 297-306
Efficacy and Safety of Orelabrutinib for Previously Treated Marginal Zone Lymphoma
Shi Lv Chena, Yu Fenga, b, Jiao Xuea, Shan Wanga, Shao Ling Wua, c
aDepartment of Hematology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
bDepartment of Geriatrics, Deyang Hospital Affiliated to Chengdu University of Traditional Chinese Medicine, Sichuan, China
cCorresponding Author: Shao Ling Wu, Department of Hematology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Manuscript submitted October 28, 2025, accepted December 10, 2025, published online December 30, 2025
Short title: Orelabrutinib for Previously Treated MZL
doi: https://doi.org/10.14740/jh2149
| Abstract | ▴Top |
Background: This study aimed to investigate the efficacy and safety of orelabrutinib monotherapy in Chinese patients with marginal zone B-cell lymphoma (MZL).
Methods: We conducted a retrospective analysis of MZL patients treated with orelabrutinib monotherapy (n = 30) or rituximab/rituximab plus bendamustine (R/BR) (n = 81) at the Affiliated Hospital of Qingdao University from January 1, 2019, to December 31, 2023. Orelabrutinib monotherapy was assigned to the experimental group. Propensity score matching (PSM) was applied to match 30 MZL patients treated with the R/BR regimen as a control group, comparing the efficacy and safety between the two groups.
Results: In both the orelabrutinib group and the control group, patients who developed relapsed or refractory (R/R) disease after non-rituximab/rituximab-based chemotherapy (non-R/R-CT) therapies (including anti-infective treatment and localized radiotherapy) achieved clinical responses. For 17 patients with R/R disease following R/R-CT treatment, no statistically significant differences were observed between the orelabrutinib group and the BR group in disease control rate (DCR) (82.35% vs. 88.23%, P = 1.00), overall response rate (ORR) (64.70% vs. 76.47%, P = 0.70), and complete response (CR) (17.64% vs. 29.41%, P = 0.68). There was no statistically significant difference in 1-year event-free survival (EFS) (86.66% (95% confidence interval (CI): 68.27 - 94.77) vs. 83.33% (95% CI: 64.49 - 92.70), P = 0.66). Hematologic adverse events (66.66% vs. 86.66%, P = 0.10), lymphopenia (6.66% vs. 40.00%, P < 0.01), and grade 3/4 adverse events (10.00% vs. 33.33%, P = 0.03) were all lower in the orelabrutinib group compared to the control group. Neither group had patients who discontinued treatment due to adverse reactions.
Conclusion: Orelabrutinib monotherapy in MZL possesses favorable efficacy and safety in our study. Given the limited sample size, further clinical research with larger cohorts and extended follow-up is necessary.
Keywords: Lymphoma; B cell; Marginal zone; Bruton’s tyrosine kinase; Orelabrutinib; Safety
| Introduction | ▴Top |
Marginal zone B-cell lymphoma (MZL) is an indolent subtype of non-Hodgkin’s lymphoma originating from the marginal zone of lymphoid tissue. It can be classified into three subtypes based on location: extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (EMZL/MALT), nodal marginal zone B-cell lymphoma (NMZL), and splenic marginal zone B-cell lymphoma (SMZL) [1]. Different types of MZL are treated in various ways. Anti-infective therapy is preferred for SMZL patients with hepatitis C virus (HCV) infection or EMZL with Helicobacter pylori (HP). For asymptomatic patients with stage III/IV disease, observation can be considered [2, 3]. According to the latest National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines [4, 5], first-line treatment options for stage I/II MZL patients who fail anti-infection therapy typically involve rituximab or radiotherapy. For SMZL, a splenectomy is a viable option. For stage III/IV and relapsed or refractory (R/R) patients, treatment options primarily include bendamustine combined with rituximab (BR) or rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). While most patients achieve remission and enjoy a high long-term survival rate, approximately 20% experience recurrence or progression within 2 years, with a median survival of only 3 - 5 years for those affected [6]. Additionally, rituximab-based chemotherapy (R-CT) is linked to a high incidence of treatment-related adverse events (TRAEs), necessitating periodic hospitalization [7]. It is crucially essential to explore further and optimal treatment protocols.
Bruton’s tyrosine kinase inhibitors (BTKis) can suppress B cells’ proliferation, differentiation, and apoptosis. Acalabrutinib and zanubrutinib have been recognized by NCCN as second-line treatment options for MZL. Orelabrutinib, a newly developed oral BTKi in China, demonstrates enhanced target selectivity and reduced off-target effects, showing promising efficacy and safety profiles in various mature B-cell lymphomas [8]. In a recent phase II multicenter study, orelabrutinib monotherapy achieved an overall response rate (ORR) of 58.9% in Chinese patients with R/R MZL, with 1-year progression-free survival (PFS) and overall survival (OS) rates of 82.8% and 91%, respectively. Notably, 30.6% of patients experienced grade 3 or higher adverse events [9]. However, most patients had experienced at least two prior lines of therapy failures in the study. Orelabrutinib has been approved in China to treat patients with MZL who have undergone at least one prior therapy. The real-world efficacy and safety of orelabrutinib still need to be further validated through multicenter clinical applications. This study aimed to retrospectively assess the efficacy and safety of orelabrutinib monotherapy in Chinese MZL patients within a single center, comparing outcomes with those treated with rituximab/rituximab plus bendamustine (R/BR).
| Materials and Methods | ▴Top |
Clinical data
We conducted a retrospective study at the Affiliated Hospital of Qingdao University on MZL patients who received orelabrutinib monotherapy or R/BR between January 1, 2019, and December 31, 2023. The inclusion criteria involved a diagnosis of MZL, confirmed through biopsy, immunohistochemistry, or next-generation sequencing; R/R or intolerant patients following prior treatments (anti-HP therapy, HCV treatment, or radiotherapy or rituximab/rituximab-based chemotherapy (R/R-CT) treatment) in the orelabrutinib group; comprehensive clinical data, including general conditions, laboratory tests, imaging studies, and follow-up; exclusion of central nervous system involvement or concurrent malignancies. This study obtained approval from the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 28901). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Stage and prognosis
Staging
Gastric and intestinal EMZL followed the modified Lugano staging system [10], while NMZL and SMZL adhered to the Lugano staging system (version 2014) [11].
Prognosis
EMZL employed the Mucosa-Associated Lymphoid Tissue Lymphoma Prognostic Index (MALT-IPI) (age, Ann Arbor staging, and lactate dehydrogenase (LDH) levels) [12], SMZL utilized the hemoglobin, platelets, LDH, and hepatosplenomegaly (HPLL) prognostic model [13]. NMZL utilized the Follicular Lymphoma International Prognostic Index (FLIPI) to stratify patients into low- and high-risk categories.
Treatments
Orelabrutinib
A dose of 150 mg was taken orally once daily until either disease progression, intolerable toxicity occurs, or discontinuation due to patient preference.
R regimen
Rituximab was administered at 375 mg/m2 by intravenous infusion once weekly for 4 - 8 consecutive weeks per treatment course.
BR regimen
Bendamustine was administered at 90 mg/m2 on days 1 - 2 and rituximab at 375 mg/m2 on day 1, both via intravenous infusion, every 28 days for four to six cycles.
In the control cohort, rituximab monotherapy was mainly used in patients with limited-stage (Lugano I/II) with relatively low tumor burden, whereas the BR regimen was preferentially administered to patients with advanced-stage (Lugano III/IV) or higher-risk disease, in accordance with contemporary NCCN and CSCO guideline-based practice.
Endpoint and assessment
Primary endpoint
In patients with MZL, event-free survival (EFS) was defined as the time from treatment initiation to disease relapse, progression, death from any cause, or treatment discontinuation due to any cause.
Secondary endpoints
1) Efficacy endpoints
Response was assessed according to the 2014 Lugano criteria [11] and the 2020 European Society for Medical Oncology (ESMO) Clinical Practice Guidelines [3] using positron emission tomography/computed tomography (PET/CT) or CT at predefined time points, combined with bone marrow evaluation or gastroscopy/colonoscopy when clinically indicated, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). ORR was calculated as CR + PR. Disease control rate (DCR) was defined as CR + PR + SD. Time to response (TTR) was defined as the interval from treatment initiation to the first documented response. R/R status included disease recurrence after initial response, progression during therapy, or failure to achieve response after a defined treatment course. OS was defined as the time from treatment initiation to death from any cause.
2) Safety endpoints
TRAEs were evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0).
Follow-up
All patients were followed until the study endpoint or June 30, 2025. Patients receiving orelabrutinib monotherapy underwent monthly complete blood count (CBC) tests and comprehensive disease assessments every 3 - 6 months. Patients receiving R/BR therapy underwent follow-up evaluations every 3 - 6 months within the first year post-immunochemotherapy, followed by annual evaluations thereafter.
Follow-up data were primarily collected through outpatient visits, hospital admissions, and remote communication, including laboratory tests, imaging studies, and documentation of symptoms and signs.
Statistical analysis
Clinical data were analyzed using SPSS 25.0, RStudio, and GraphPad Prism 9.5.1 software. Orelabrutinib monotherapy was assigned to the experimental group, and PSM at a 1:1 ratio was utilized to match MZL patients treated with the R/BR regimen as the control group. The caliper value was set to 0.3. PSM covariates include gender, age, treatment timing, subtype, stage, prognosis, Ki-67 index, bone marrow involvement, and Eastern Cooperative Oncology Group (ECOG) performance status. The standardized mean difference (SMD) was calculated using Hedges’ g method after matching. Given the small sample size, an SMD of less than 0.15 was set as the threshold for covariate balance between the two groups.
Normally distributed data were presented as mean (standard deviation), while skewed data were described using median (range). Depending on the circumstances, between-group differences were assessed using either the Mann-Whitney U test or the Fisher’s exact test. Survival analysis employed the Kaplan-Meier method, between-group survival curves were compared using the Log-rank test, and pointwise 95% confidence intervals (95% CIs) for survival probabilities were calculated using Greenwood’s formula with the log-log transformation. A significance level of P < 0.05 indicates statistical significance for differences between groups.
| Results | ▴Top |
Patients
As of December 31, 2023, our center initiated monotherapy with orelabrutinib for 30 patients diagnosed with MZL. Among them, the average age was 61.03 (10.07) years, and the median age was 60 years (35 - 80); 19 (63.33%) were male. Among the cases, 15 (50.00%) were EMZL, including 10 (33.33%) cases of gastrointestinal MALT, nine (30.00%) were NMZL, and six (20.00%) were SMZL. Twenty-four (80.00%) were classified as stage III/IV with intermediate or high risk. Of these patients, 17 (56.66%) had R/R disease after R/R-CT, of which 16 were stage III/IV. Among these 17 cases, nine experienced relapses (eight at first relapse), and eight were refractory, including four who failed to respond to first-line therapy and four who progressed during treatment, with prior treatments consisting of rituximab alone in 10 patients, R-CHOP in two patients, and rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) in five patients. The remaining 13 patients included seven who were resistant to anti-HP, four who had failed after radiotherapy, and two who were intolerant to rituximab.
Additionally, 81 MZL patients with the R/BR regimen showed statistically significant differences in prior therapy compared to the orelabrutinib group (P < 0.05) (Table 1, before PSM). A matched control group of 30 MZL patients treated with the R/BR regimen was obtained through PSM (1:1), among whom 17 (56.66%) patients experienced R/R disease after R/R-CT, all classified as stage III/IV. Of these 17 cases, 11 experienced first relapse and six were refractory after first-line therapy, which consisted of rituximab alone in eight patients, R-CVP in six patients, and R-CHOP in three patients. The remaining 13 patients included seven who failed HP eradication therapy, three who progressed after radiotherapy, and three treatment-naive cases. Additionally, 15 patients had EMZL, with nine (60.00%) involving gastrointestinal MALT. Post-matching baseline characteristics between the two groups showed no significant differences (P > 0.05) and achieved adequate balance (SMD < 0.15) (Table 2, after PSM).
 Click to view | Table 1. Before PSM |
 Click to view | Table 2. After PSM |
Efficacy
As of June 30, 2025, the orelabrutinib group had a median follow-up of 20.95 (6.00 - 45.60) months, with a mean treatment duration of 20.44 (6.92) months and a median treatment duration of 16.00 (6.00 - 45.60) months. The mean time to response (TTR) was 6.07 (1.59) months, and the median TTR was 6.00 (3.00 - 9.00) months. Notably, one intermediate-risk NMZL patient treated with orelabrutinib remained free of recurrence or progression after 41.60 months of follow-up, and five stage I/II patients (three gastrointestinal EMZL, one thyroid EMZL, and one pulmonary EMZL) achieved remission within 4 months.
In the control group, the median follow-up was 25.55 (2.00 - 62.80) months, with a mean of 6.16 (1.78) treatment cycles received and a median of 6.00 (2.00 - 10.00) treatment cycles. Five stage I/II EMZL patients received rituximab monotherapy with a mean treatment duration of 6.2 (1.1) weeks, and 25 stage III/IV EMZL patients received BR with a mean of 5.9 (2.5) cycles, among whom five patients underwent an average of four cycles of rituximab consolidation therapy following BR treatment.
No statistically significant differences were observed between the orelabrutinib group and the control group in DCR (90.00% vs. 93.33%, P = 1.00), ORR (80.00% vs. 86.66%, P = 0.73), and CR rate (30.00% vs. 53.33%, P = 0.11) (Fig. 1). All stage I/II patients and non-R/R-CT-treated R/R patients achieved remission in both groups. Among R/R-CT-treated R/R patients, comparisons of DCR (82.35% vs. 88.23%, P = 1.00), ORR (64.70% vs. 76.47%, P = 0.70), and CR rate (17.64% vs. 29.41%, P = 0.68), as well as subtype-specific analyses (SMZL, NMZL, EMZL), showed no statistically significant differences (Table 3).
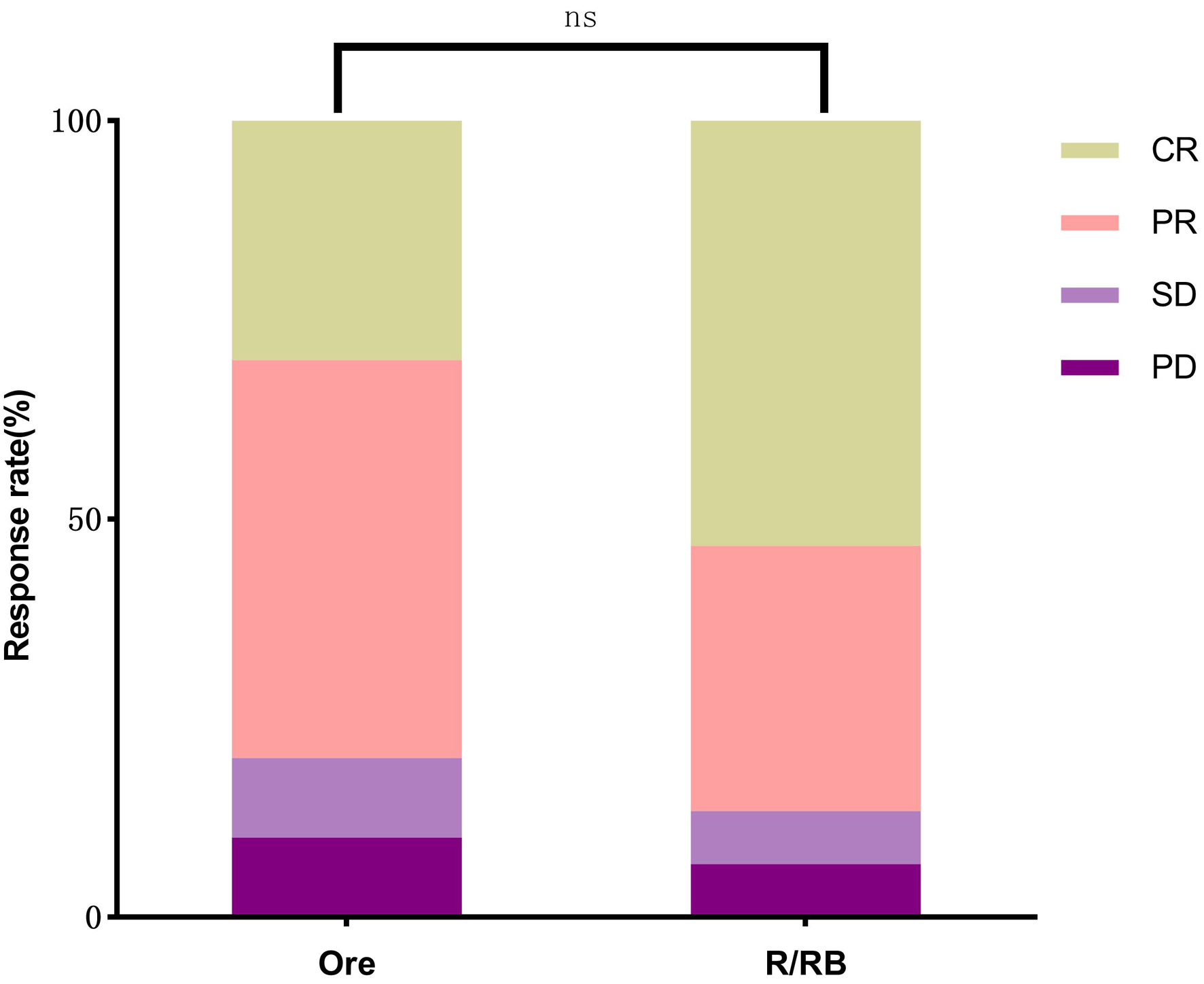 Click for large image | Figure 1. Comparison of CR (30.00% vs. 53.3%), PR (50.00% vs. 33.33%), SD (10.00% vs. 6.66%), and PD (10.00% vs. 6.66%) between the orelabrutinib and control groups (P > 0.05). CR: complete response; PD: progressive disease; PR: partial response; SD: stable disease. |
 Click to view | Table 3. Comparison of DCR, ORR, CR, and PR Between Relapsed/Refractory Patients After R/R-CT in the Two Groups (P > 0.05) |
The overall 1-year EFS rate (86.66% (95% CI: 68.27 - 94.77) vs. 83.33% (95% CI: 64.49 - 92.70), P = 0.66) and the 1-year EFS rate in R/R-CT-treated patients (76.47% (95% CI: 48.83 - 90.45) vs. 70.58% (95% CI: 43.15 - 86.56), P = 0.59) showed no statistical differences between the two groups (Fig. 2). In the orelabrutinib group, one SMZL, one NMZL, and one EMZL patient failed to achieve response after nearly 1 year of treatment, while one NMZL and two SMZL patients progressed at 6, 7.6, and 13 months, respectively. The control group showed early progression during treatment in one EMZL and one NMZL patient; failure to achieve treatment response in one SMZL and one NMZL patient; relapse at 12, 29, 30, and 40 months post-remission in one NMZL, one SMZL, one NMZL, and one EMZL patient, respectively; and one elderly patient death from acute cardio-cerebrovascular events at 12 months post-treatment.
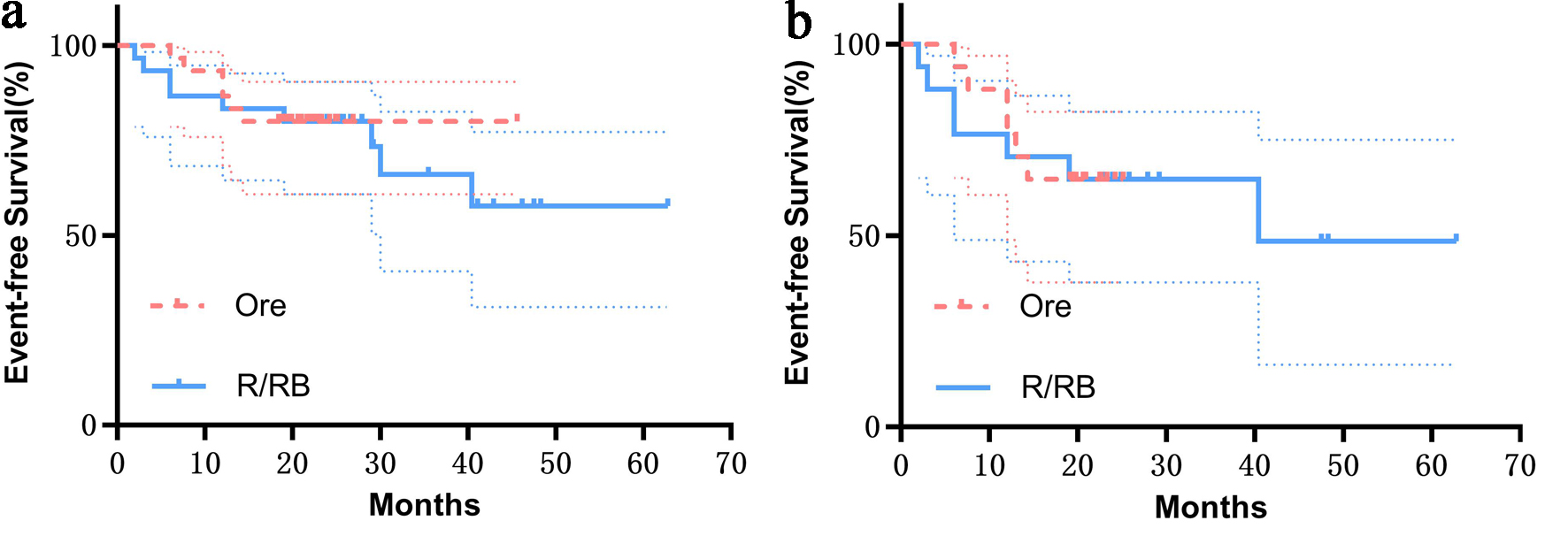 Click for large image | Figure 2. (a) Overall EFS of the two groups (P = 0.86). (b) EFS of patients after R/R-CT treatment (P = 0.75). Shaded bands denote 95% confidence intervals, and no significant statistical differences were observed in either case. EFS: event-free survival; Ore: orelabrutinib; R/BR: rituximab/bendamustine plus rituximab; R/R-CT: rituximab/rituximab-based chemotherapy. |
Safety
In the orelabrutinib group, 26 patients (86.66%) experienced TRAEs. The most common TRAEs were hematologic adverse events (n = 20, 66.66%), including thrombocytopenia in eight cases (26.66%), neutropenia in six cases (20.00%), anemia in four cases (13.33%), and leukopenia or lymphopenia in three cases (10.00%). Grade 3 neutropenia or thrombocytopenia was observed in three cases (10.00%), with the remaining TRAEs categorized as grade 1/2. Non-hematologic adverse events included grade 2 pneumonia or upper respiratory infection with fever in three cases (10.00%). The pneumonia case was complicated by grade 3 neutropenia, which resolved after initiating anti-infective therapy. Rash occurred in five cases (16.66%) and hematuria in two cases (6.66%), all classified as grade 1 or 2 TRAEs. No cardiac arrhythmias or other TRAEs were observed (Table 4).
 Click to view | Table 4. TRAEs of Orelabrutinib Group |
Twenty-nine (96.66%) patients experienced TRAEs in the control group, hematologic adverse events were observed in 26 cases (86.66% vs. 66.66%, P = 0.10), lymphopenia in 12 cases (40.00% vs. 6.66%, P < 0.01), and grade 3/4 TRAEs in 10 cases (33.33% vs. 10.00%, P = 0.03), all of which were numerically higher compared to the orelabrutinib group, with lymphopenia and grade 3/4 TRAEs showing statistically significant differences. Grade 3/4 TRAEs included lymphopenia in six cases (20.00%) and neutropenia in four cases (13.33%); of these patients, five patients (16.66%) experienced lymphopenia or neutropenia with grade 3 pneumonia or upper respiratory infection. Other grade 1/2 TRAEs included anorexia in six cases (20.00%), lymphopenia in four cases (20.00%), nausea/vomiting in four cases (13.33%), neutropenia, thrombocytopenia, and pneumonia in two cases each (6.66%), and atrial fibrillation in one case (3.33%) (Fig. 3). Treatment was not discontinued in either group due to TRAEs.
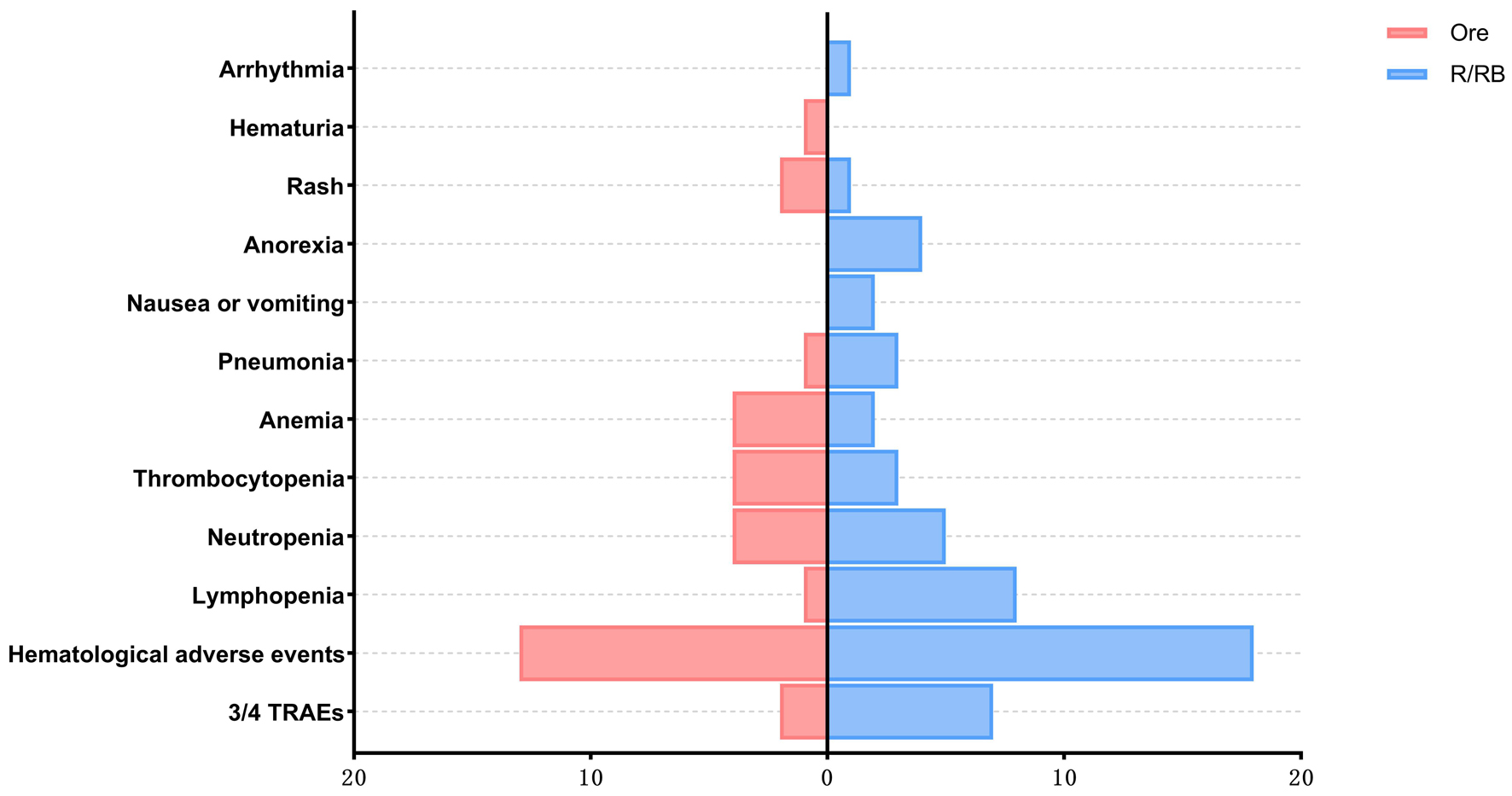 Click for large image | Figure 3. The numbers of adverse events in the two groups. 3/4 TRAE: grade 3/4 treatment-related adverse event. PSM: propensity score matching; R/BR: rituximab/bendamustine plus rituximab. |
| Discussion | ▴Top |
This real-world study compares the treatment outcomes of 30 Chinese patients with MZL treated with orelabrutinib monotherapy with R/BR regimens through PSM. Clinical trials further confirm orelabrutinib’s efficacy and safety.
The majority of MZL patients are middle-aged and elderly [14]. Among the 111 patients in this study, the median age was 59 years (range 30 - 80), predominantly male. For patients with indolent B-cell lymphomas such as MZL, optimizing treatment hinges on disease control, minimizing side effects, and enhancing quality of life. However, the current immunochemotherapy regimens may not be optimal for MZL. Two retrospective studies of bendamustine as initial therapy for MZL showed high response rates and prolonged PFS. However, grade 3/4 TRAEs were reported at 58.3% and 75%, respectively. The most frequent TRAEs were hematologic toxicities, primarily lymphocyte and neutrophil reductions [15, 16], mirroring findings in our control group. R-CT regimens generally manifest higher rates of TRAEs. In the BRIGHT study, R-CHOP/CVP exhibited elevated rates of alopecia, nausea, and vomiting, along with neurotoxicity from cyclophosphamide and cardiotoxicity from vincristine/doxorubicin compared to BR. Moreover, grade 3/4 hematologic adverse events were more prevalent with R-CHOP/CVP than with BR [17].
Faced with higher side effects of R-CT, BTKi presents a suitable option. Ibrutinib demonstrated a DCR of 83% and an ORR of 48% with a CR rate of 3% in a phase II trial of R/R MZL, although treatment discontinuation due to TRAEs occurred in 19% of patients [18]. Zanubrutinib and orelabrutinib, as next-generation BTKis with improved target selectivity, were developed to enhance efficacy while mitigating off-target toxicities. In a phase II study of zanubrutinib for R/R MZL, the DCR was 87.9%, with an ORR of 68.2% and a CR rate of 25.8%; the most common TRAEs were neutropenia (8.8%) and pneumonia (10.3%), and 6% of patients discontinued treatment due to TRAEs [19]. In a phase II trial of orelabrutinib monotherapy in R/R MZL, the ORR and CR rates were comparable to those of zanubrutinib, with a favorable safety profile, likely due to differences in sample size and assessment methods, yet both were superior to ibrutinib [9].
In the aforementioned clinical trials, most patients with R/R had received at least one prior R/R-CT treatment. In our cohort, the orelabrutinib group comprised 17 cases of R/R after R/R-CT, achieving a DCR of 82.35% and an ORR of 64.70%. Specifically, the ORR for SMZL was the lowest at 25%, with no patients achieving CR. The ORR observed in the aforementioned prospective study of orelabrutinib was slightly lower than in our study, primarily because 61% of patients received ≥ 2 prior therapies in that study, whereas most patients in our orelabrutinib group had received only one prior treatment. Regardless of the research or other BTKi studies, the ORR for SMZL remains relatively low. As a distinct subtype within the MZL spectrum, SMZL exhibits increased invasiveness, with approximately 80% of patients showing bone marrow involvement. Its 10-year OS is lower than the MALT subtype (57.9% vs. 81.4%) [20]. Combining BR with orelabrutinib can be considered to enhance the overall depth of remission in SMZL and MZL patients. Yu et al [21] demonstrated through cell and murine models that combining orelabrutinib with rituximab enhances apoptosis in B-cell lymphoma and sustains natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC).
Moreover, the chemoimmunotherapy group showed a numerically higher CR rate in our cohort, whereas the orelabrutinib group had a higher proportion of PR with comparable ORR and DCR. This pattern is broadly consistent with the known response profile of BTKis, which often induce durable PRs with gradual deepening over time. Given the limited sample size and follow-up, the observed difference in CR rates did not reach statistical significance and should be interpreted with caution.
Regarding safety, multiple clinical studies have defined the common TRAEs of orelabrutinib well. According to existing literature reports [22], the main adverse effects of this agent can be categorized into hematologic toxicity and non-hematologic toxicity: the former includes thrombocytopenia (20-35%), neutropenia (15-25%), and anemia (10-18%); the latter (> 10% incidence) primarily manifests as rash, respiratory tract infections, hematuria, arrhythmia, and hypertension. Notably, the TRAEs observed in this study were broadly consistent with the known safety profile but exhibited milder clinical manifestations. Hematologic toxicity remained the predominant adverse reaction type, with thrombocytopenia accounting for the majority. However, 85% of hematologic events were grade 1/2, and only three cases of grade 3 thrombocytopenia occurred, with none significantly impacting patients’ quality of life. Regarding non-hematologic toxicities, the rash was most frequently observed, while other BTKi-associated special TRAEs, such as blood pressure elevation, atrial fibrillation, or hemorrhage other than hematuria, were not detected.
Additionally, from an economic perspective, the average cost for one cycle of Mabthera for a normal-sized adult patient at our center is approximately $1,401, with bendamustine costing around $206, and after reimbursement, roughly $275 per month for orelabrutinib. When considering overall hospitalization expenses, orelabrutinib monotherapy proves to be more economical.
In conclusion, orelabrutinib monotherapy demonstrates favorable efficacy and safety in MZL management, particularly for patients with stage I/II disease or those without prior R/R-CT exposure, who exhibited deeper therapeutic responses compared to R/R-CT pretreated individuals. BTKi monotherapy may serve as a viable first-line option for low-to-intermediate risk MZL patients or those with limited tumor burden. In a prospective trial of ibrutinib monotherapy in treatment-naive MZL patients [23], predominantly with stage III/IV disease, an ORR of 88% was achieved, accompanied by 1- and 3-year PFS rates of 83.3% and 55.6%, respectively.
Notably, this study has several limitations, including a relatively small sample size, single-center retrospective design, and incomplete documentation of TRAEs, all of which may lead to underestimation of some adverse events. In addition, the control group combined rituximab monotherapy and BR, which reflects real-world, guideline-driven practice patterns rather than a single protocolized regimen. Although PSM was used to balance key baseline characteristics, residual confounding related to regimen selection cannot be fully excluded and should be interpreted as an inherent limitation of this real-world design. During the current follow-up, only one death was documented, and OS data were therefore immature; accordingly, we focused on EFS as the more informative time-to-event endpoint in this cohort. The relatively short follow-up precludes definitive conclusions regarding long-term outcomes, optimal treatment duration, and durability of response after discontinuation. Therefore, these findings should be interpreted with caution and warrant confirmation in larger, prospective multicenter studies. Future research should prioritize exploring rational combinations of orelabrutinib with immunochemotherapy or other targeted agents to further enhance therapeutic efficacy and overcome resistance mechanisms in MZL [24].
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents (consent to participate and consent to publish) were obtained from all participants.
Author Contributions
Shi Lv Chen: data curation, resources, software, validation, investigation, methodology, Writing – original draft, writing – review and editing. Yu Feng: data curation, formal analysis, investigation, visualization, writing – original draft. Jiao Xue: software, formal analysis, investigation, visualization, writing – original draft. Shan Wang: formal analysis, software, investigation, writing – original draft. Shao Ling Wu (corresponding author): conceptualization, resources, supervision, funding acquisition, methodology, project administration, writing – original draft, writing – review and editing.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author, Shao Ling Wu, upon reasonable request.
Abbreviations
ADCC: antibody-dependent cellular cytotoxicity; BTK: Bruton tyrosine kinase; BTKi: Bruton tyrosine kinase inhibitor; CBC: complete blood count; CI: confidence interval; CR: complete response; CSCO: Chinese Society of Clinical Oncology; CT: computed tomography; CTCAE: Common Terminology Criteria for Adverse Events; CVP: cyclophosphamide, vincristine, prednisone; DCR: disease control rate; ECOG: Eastern Cooperative Oncology Group; EFS: event-free survival; EMZL: extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue; ESMO: European Society for Medical Oncology; FLIPI: Follicular Lymphoma International Prognostic Index; HCV: hepatitis C virus; HP: Helicobacter pylori; HPLL: hemoglobin, platelets, lactate dehydrogenase, and hepatosplenomegaly; Ki-67: Ki-67 proliferation index; LDH: lactate dehydrogenase; MALT: mucosa-associated lymphoid tissue; MALT-IPI: MALT Lymphoma Prognostic Index; MZL: marginal zone lymphoma; NCCN: National Comprehensive Cancer Network; NK: natural killer; NMZL: nodal marginal zone lymphoma; ORR: overall response rate; OS: overall survival; PD: progressive disease; PFS: progression-free survival; PR: partial response; PSM: propensity score matching; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP: rituximab, cyclophosphamide, vincristine, and prednisone; R/R: relapsed or refractory; R/R-CT: rituximab/rituximab-based chemotherapy; R/BR: rituximab or rituximab plus bendamustine; BR: rituximab plus bendamustine; SD: stable disease; SMD: standardized mean difference; SMZL: splenic marginal zone lymphoma; TRAEs: treatment-related adverse events; TTR: time to response
| References | ▴Top |
- Cerhan JR, Habermann TM. Epidemiology of marginal zone lymphoma. Ann Lymphoma. 2021;5.
doi pubmed - Cheah CY, Seymour JF. Marginal zone lymphoma: 2023 update on diagnosis and management. Am J Hematol. 2023;98(10):1645-1657.
doi pubmed - Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, Ricardi U, et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(1):17-29.
doi pubmed - National Comprehensive Cancer Network. NCCN clinical practice guidelines in B-cell lymphoma (Version 1.2024). nccn.org.
- Chinese Society of Clinical Oncology. Lymphoid malignancies. 2024. CSCO.org.
- Rossi D, Bertoni F, Zucca E. Marginal-zone lymphomas. N Engl J Med. 2022;386(6):568-581.
doi pubmed - Salar A, Domingo-Domenech E, Panizo C, Nicolas C, Bargay J, Muntanola A, Canales M, et al. Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma. Blood. 2017;130(15):1772-1774.
doi pubmed - Song Y, Wu SJ, Shen Z, Zhao D, Chan TSY, Huang H, Qiu L, et al. Chinese expert consensus on Bruton tyrosine kinase inhibitors in the treatment of B-cell malignancies. Exp Hematol Oncol. 2023;12(1):92.
doi pubmed - Deng L, Li Z, Zhang H, Huang H, Hu J, Liu L, Liu T, et al. Orelabrutinib for the treatment of relapsed or refractory marginal zone lymphoma: a phase 2, multicenter, open-label study. Am J Hematol. 2023;98(11):1742-1750.
doi pubmed - Ruskone-Fourmestraux A, Dragosics B, Morgner A, Wotherspoon A, De Jong D. Paris staging system for primary gastrointestinal lymphomas. Gut. 2003;52(6):912-913.
doi pubmed - Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
doi pubmed - Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, Martelli M, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409-1417.
doi pubmed - Montalban C, Abraira V, Arcaini L, Domingo-Domenech E, Guisado-Vasco P, Iannitto E, Mollejo M, et al. Simplification of risk stratification for splenic marginal zone lymphoma: a point-based score for practical use. Leuk Lymphoma. 2014;55(4):929-931.
doi pubmed - Bron D, Meuleman N. Marginal zone lymphomas: second most common lymphomas in older patients. Curr Opin Oncol. 2019;31(5):386-393.
doi pubmed - Wang YW, Xu JD, Maihemaiti A, et al. Efficacy and safety of bendamustine plus rituximab in Chinese de novo margin zone lymphoma patients: a multicenter retrospective study based on propensity score matching. J Clin Hematol (China). 2022,35(01).
- Morigi A, Argnani L, Lolli G, Broccoli A, Pellegrini C, Nanni L, Stefoni V, et al. Bendamustine-rituximab regimen in untreated indolent marginal zone lymphoma: experience on 65 patients. Hematol Oncol. 2020;38(4):487-492.
doi pubmed - Flinn IW, van der Jagt R, Kahl B, Wood P, Hawkins T, MacDonald D, Simpson D, et al. First-line treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2019;37(12):984-991.
doi pubmed - Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, Collins GP, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224-2232.
doi pubmed - Opat S, Tedeschi A, Hu B, Linton KM, McKay P, Leitch S, Coleman M, et al. Safety and efficacy of zanubrutinib in relapsed/refractory marginal zone lymphoma: final analysis of the MAGNOLIA study. Blood Adv. 2023;7(22):6801-6811.
doi pubmed - Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119(3):629-638.
doi pubmed - Yu H, Wang X, Li J, Ye Y, Wang D, Fang W, Mi L, et al. Addition of BTK inhibitor orelabrutinib to rituximab improved anti-tumor effects in B cell lymphoma. Mol Ther Oncolytics. 2021;21:158-170.
doi pubmed - Dhillon S. Orelabrutinib: First Approval. Drugs. 2021;81(4):503-507.
doi pubmed - Epperla N, Zhao Q, Moyo T, Watkins MP, Tavakkoli M, Bello C, Torka P, et al. Outcomes of marginal zone lymphoma treated with ibrutinib in the first-line setting in the United States: a real-world analysis. Blood Adv. 2024;8(3):549-552.
doi pubmed - Wang H, Zhang W, Yang J, Zhou K. The resistance mechanisms and treatment strategies of BTK inhibitors in B-cell lymphoma. Hematol Oncol. 2021;39(5):605-615.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.