| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 6, December 2025, pages 314-323
Efficacy and Safety of Daratumumab, Lenalidomide and Dexamethasone Therapy in the First Relapse of Multiple Myeloma Patients – Real World Data from Hungary
Szilvia Lovasa, b, Nora Obajed Al-Alia, b, Katalin Farkasa, b, Laszlo Imre Pinczesa, b, Gergely Vargac, Gabor Mikalac, Peter Rajnicsd, Szabolcs Kosztolanyie, Mark Planderf, Piroska Pettendig, Laszlo Varoczya, b, h
aDepartment of Hematology, Institute for Medicine, Faculty of Medicine, University of Debrecen, Debrecen, Hungary
bDoctoral School of Clinical Sciences, University of Debrecen, Debrecen, Hungary
cDepartment of Medicine and Hematology, Faculty of Medicine, Semmelweis University, Budapest, Hungary
dDepartment of Hematology, Mor Kaposi Hospital, Kaposvar, Hungary
e1st Department of Medicine, Faculty of Medicine, University of Pecs, Pecs, Hungary
fDepartment of Hematology and Hemostasis, Markusovszky Hospital, Szombathely, Hungary
g1st Department of Medicine, Geza Hetenyi Hospital, Szolnok, Hungary
hCorresponding Author: Laszlo Varoczy, Department of Hematology, Institute for Medicine, University of Debrecen, H-4032 Debrecen, Hungary
Manuscript submitted October 8, 2025, accepted November 21, 2025, published online December 30, 2025
Short title: D-Rd in MM
doi: https://doi.org/10.14740/jh2142
| Abstract | ▴Top |
Background: Daratumumab is a monoclonal antibody targeting CD38, which has demonstrated efficacy in both newly diagnosed and relapsed or refractory multiple myeloma (MM). The objective of our study was to evaluate the effectiveness and safety of the daratumumab, lenalidomide, and dexamethasone (D-Rd) regimen as a second-line therapy in a real-world clinical setting.
Methods: A total of 55 Hungarian patients with MM were enrolled, all of whom received D-Rd at first relapse through a special reimbursement program between February 2022 and August 2023. Treatment responses, progression-free survival (PFS), and overall survival (OS) were assessed in the intent-to-treat population as well as in selected subgroups. Safety outcomes were also systematically collected.
Results: Treatment response was observed in 49 patients (89%), with 12 complete responses, 22 very good partial responses, and 15 partial responses. After a median follow-up of 36.6 months, the median PFS for the entire cohort was 22.0 months, while median OS had not been reached yet. Patients with advanced-stage disease (Revised International Staging System stage 3) showed significantly poorer survival outcomes compared with those in stage 1 or 2 (PFS: P < 0.001; OS: P = 0.015). High-risk cytogenetic abnormalities and frailty were also associated with markedly inferior prognosis. In contrast, neither prior autologous stem cell transplantation nor lenalidomide maintenance therapy had a significant impact on survival. During the study period, three deaths due to severe infections occurred, while the most common adverse events were mild hematologic toxicities and injection-related reactions.
Conclusions: Our findings support the use of D-Rd as an effective option for first relapse in MM patients. However, our survival data are inferior to the results of the pivotal studies targeting the same population.
Keywords: Multiple myeloma; Daratumumab; Lenalidomide; Treatment response; Progression-free survival; Overal survival; Frailty
| Introduction | ▴Top |
Multiple myeloma (MM) is a type of lymphoproliferative disorder representing approximately 1% of all malignancies. It involves the uncontrolled growth of abnormal plasma cells, leading to complications such as bone destruction, elevated calcium levels, bone marrow failure, and kidney dysfunction. Although currently considered incurable, recent advances in treatment options have significantly improved patient survival rates [1]. In newly diagnosed MM, recently published guidelines recommend the use of quadruplet regimens comprising an anti-CD38 monoclonal antibody, bortezomib, lenalidomide, and dexamethasone. However, triplet combinations and other therapeutic alternatives may also be appropriate in certain clinical situations. In the relapsed setting, various combinations of anti-CD38 or anti-signaling lymphocytic activation molecule family member 7 (anti-SLAMF7) monoclonal antibodies, proteasome inhibitors, immunomodulatory drugs, and antibody-drug conjugates are available. Furthermore, bispecific antibodies and chimeric antigen receptor T-cell (CAR-T) therapies have shown the potential to improve survival outcomes in patients with refractory disease following second or subsequent relapses [2].
Daratumumab is an IgG1κ monoclonal antibody, specifically binds to CD38 and destroys plasma cells via antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Beyond immune-mediated tumor cell killing, daratumumab may also enhance immune responses by activating T cells and reducing immunosuppression [3]. Its anti-myeloma activity can be amplified when combined with immunomodulatory drugs like lenalidomide, which stimulates effector immune cells toward tumor destruction [4].
Currently, daratumumab is recognized as one of the most potent agents approved for both newly diagnosed and relapsed or refractory MM (RRMM) [5]. In the POLLUX trial, daratumumab was administered in a combination with lenalidomide and dexamethasone (D-Rd) for patients having received 1 - 9 lines of previous therapies. Recent findings from this study, which had a median follow-up period of 54.8 months, showed that the D-Rd treatment led to a 56% decrease in the likelihood of disease progression or mortality when compared to the control lenalidomide-dexamethasone (Rd) regimen. Patients receiving D-Rd experienced a median progression-free survival (PFS) of 45.0 months, significantly longer than the 17.5 months observed with Rd alone. The responses continued to improve over time, with increased rates of minimal residual disease (MRD) negativity. Among patients who had undergone only one prior therapy line, the median PFS extended to 53.3 months with D-Rd, compared to 19.6 months with Rd alone [6].
Clinical trials generally recruit patients with relatively preserved clinical status; therefore, assessing the effectiveness of novel therapeutic approaches in real-world settings remains of particular importance. In Hungary, daratumumab had previously been reimbursed solely as a third-line treatment option. In 2022, a special reimbursement framework was established, permitting its administration for a restricted number of patients at the time of first relapse. The present study aimed to investigate the efficacy and safety profile of the daratumumab-lenalidomide-dexamethasone combination as a second-line regimen in Hungarian patients with RRMM.
| Materials and Methods | ▴Top |
A retrospective review was conducted across all Hungarian centers where daratumumab-lenalidomide-dexamethasone therapy was initiated in the first relapse of MM patients between February 2022 and August 2023. Following physician referrals, a designated committee of myeloma specialists assessed patient eligibility, after which reimbursement for second-line D-Rd therapy was approved (Fig. 1). Previous lenalidomide therapy was allowed; however, lenalidomide refractory patients were excluded from the study. Patient records of individuals with MM were examined, focusing on variables such as age, gender, clinical stage, renal function, treatment response, and survival outcomes. The staging according to the Revised International Staging System (R-ISS) was applied based on criteria established by the International Myeloma Working Group (IMWG). The methodology for fluorescence in situ hybridization (FISH) testing varied among centers, with no standardized approach to probe selection; however, common targets included 17p deletion, translocations t(11;14), t(4;14), t(14;16), and 1q amplification. FISH abnormalities associated with poorer prognosis encompassed t(4;14), t(14;16), and del(17p). Frailty was assessed retrospectively for all patients using three parameters: age, the Charlson Comorbidity Index (CCI) derived from a review of each patient’s medical history, and their baseline Eastern Cooperative Oncology Group performance status (ECOG PS). Frailty status was assessed according to each patient’s age category (≤ 75, 76 - 80, or ≥ 81 years) and ECOG PS. Each age and ECOG PS category was assigned a predefined score: ≤ 75 years, 0 point; 76 - 80 years, 1 point; ≥ 81 years, 2 points; ECOG PS of 0 - 1, 0 point; and ECOG PS ≥ 2, 1 point. The sum of these two component scores defined the patient’s overall frailty status, categorized as “fit” (total score = 0), “intermediate” (total score = 1), or “frail” (total score ≥ 2). For further analysis, frailty status was also dichotomized into non-frail (scores of 0 - 1, combining fit and intermediate groups) and frail (score ≥ 2).
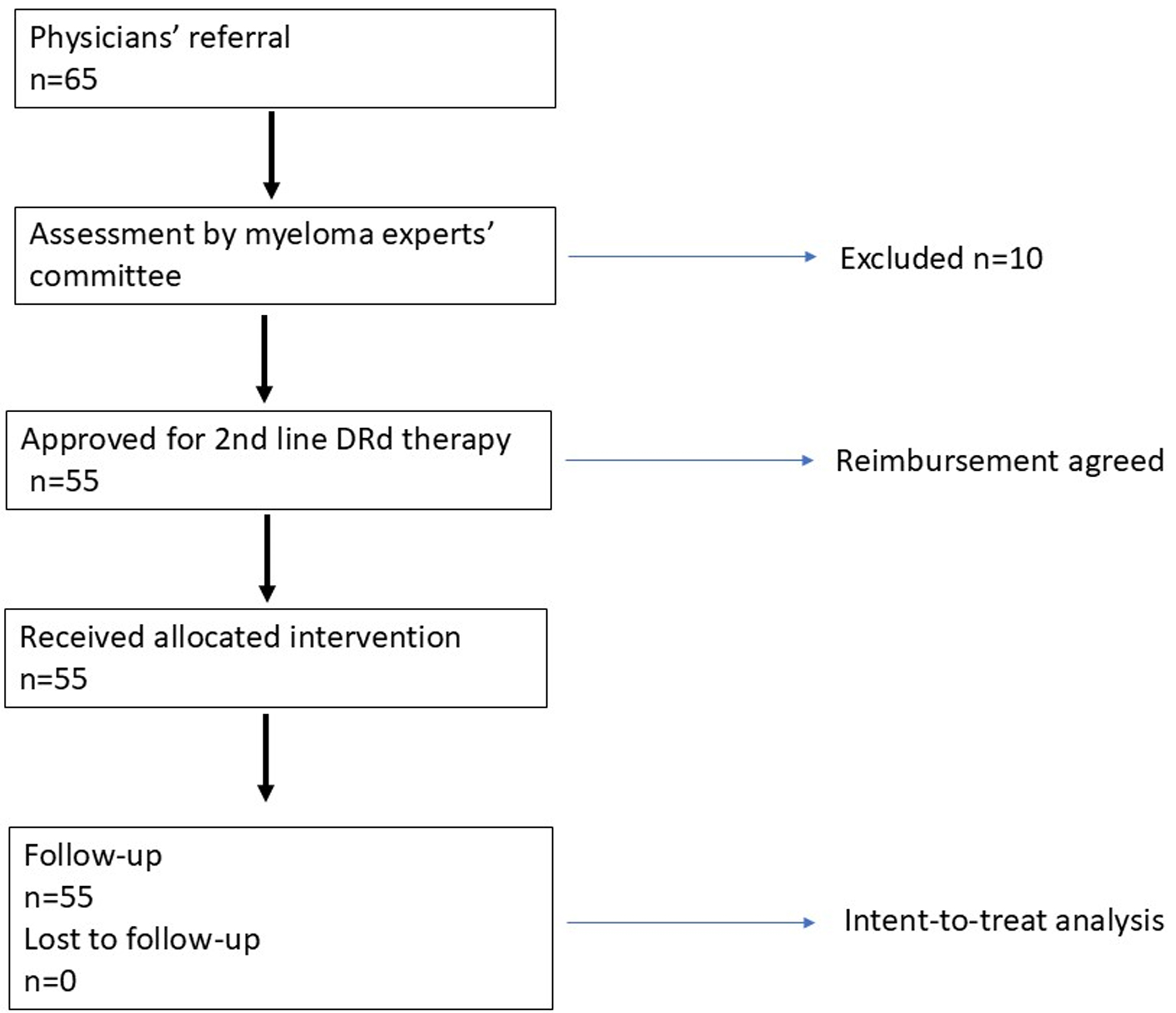 Click for large image | Figure 1. Study design. |
Patients received daratumumab at a dose of 1,800 mg administered subcutaneously once a week during the initial 8 weeks, followed by every 2 weeks thereafter. Dexamethasone at 20 mg was given either orally or intravenously on days 1, 2, 8, 9, 15, 16, and 22. Lenalidomide was administered at doses ranging from 10 to 25 mg on days 1 through 21. Dose reduction was considered if glomerular filtration rate (GFR) was < 50 mL/min or cytopenias ocurred.
The primary endpoint for efficacy was PFS. Secondary endpoints were overall response rate (ORR), rates of very good partial response (VGPR) or complete response (CR), and overall survival (OS). Treatment responses as well as survival metrics were classified according to published IMWG guidelines. OS was calculated by accounting for any death events, regardless of cause, while PFS considered relapses, death events, or disease progression warranting additional treatment. Survival data were analyzed using the Kaplan-Meier method, with comparisons made via the log-rank test. Statistical significance was defined as a P-value less than 0.05.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research project was approved by the Regional Ethical Committee of the University of Debrecen.
| Results | ▴Top |
Patient characteristics
Seven centers responded with 55 patients altogether, who were treated with daratumumab-lenalidomide-dexamethasone therapy in their first relapse between February 2022 and August 2023. Patient demographics were generally balanced, and 52.7% of the patients were female. Average age was 64.4 ± 9.6 (median: 65; range: 41 - 78). Almost all patients had prior bortezomib (98%) and 30% had thalidomide, 44% received an alkylating agent (melphalan or cyclophosphamide) and 43% underwent autologous stem cell transplantation (APSCT). Only 16% were administered lenalidomide as maintenance therapy after APSCT, but none of them were refractory to this treatment. The median time interval between the end of first-line therapy and the initiation of D-Rd was 30.4 months (4 - 62 months). Cytogenetic FISH tests of 53 patients were available, of whom 28 (52.8%) had high risk alterations. The majority had high International Staging System (ISS) score: 16, 11, and 28 were in the R-ISS 1, 2, and 3 groups, respectively (Table 1). Treatment usually continued until progression, unacceptable toxicity or death, and the median number of cycles was 24. The lenalidomide dose was reduced to 10 mg in 21 patients (38%) due to impaired renal function or the development of cytopenias.
 Click to view | Table 1. Demographic and Clinical Characteristics of the Patient Population |
Adverse events (AEs)
According to the reported number of AEs, daratumumab treatment was well tolerated. AEs above grade 1 - 2 were rare. The most frequent AEs were mild injection-associated reactions (IARs, grade 1 - 2), hematologic toxicities (mostly neutropenia), and infections.
There were three fatal infections with septicemia. Other fatal AEs were absent. Infusion-related toxicities were manageable in all cases (Table 2). The incidence of AEs was significantly higher among frail patients compared with non-frail patients (57% vs. 38%, P = 0.03).
 Click to view | Table 2. Adverse Events |
Efficacy
Response rate was assessable in all patients with 12 complete, 22 very good partial, and 15 partial responses. Six patients did not respond to the therapy or showed progressive disease.
The clinical cut-off date was August 31, 2025. At a median duration of follow-up of 36.6 months (range 25 - 43), the median PFS was 22.0 months in the whole cohort while the median OS has not been reached yet (Fig. 2). PFS and OS rates at 36 months from treatment initiation were 45.3% and 65.5%, respectively. Patients having an advanced stage disease (R-ISS 3) had significantly worse survival results than those who had stage 1 or 2 myeloma (Fig. 3). The presence of high-risk cytogenetic alterations as well as frail medical conditions also had a markedly negative impact on the patients’ prognosis (Figs. 4 and 5). Interestingly, neither the previous autologous stem cell transplantation, nor lenalidomide maintenance therapy influenced significantly the survival rates (Figs. 6 and 7).
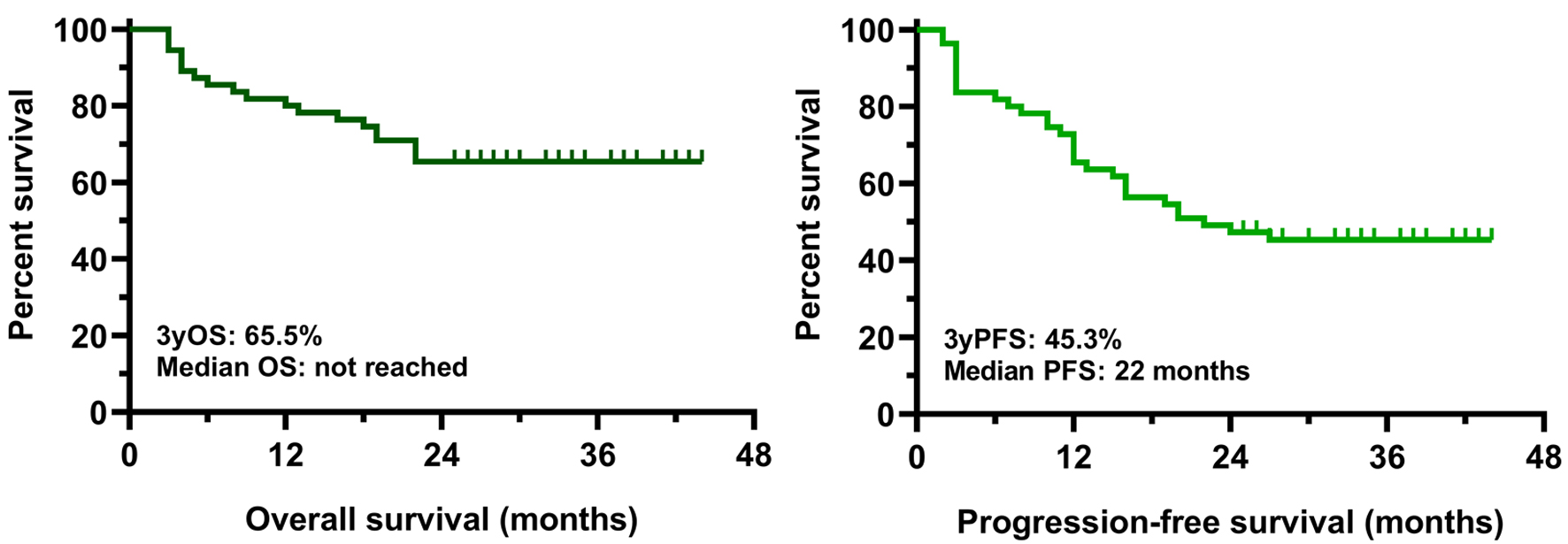 Click for large image | Figure 2. OS and PFS results in the whole intent-to-treat population. OS: overall survival; PFS: progression-free survival. |
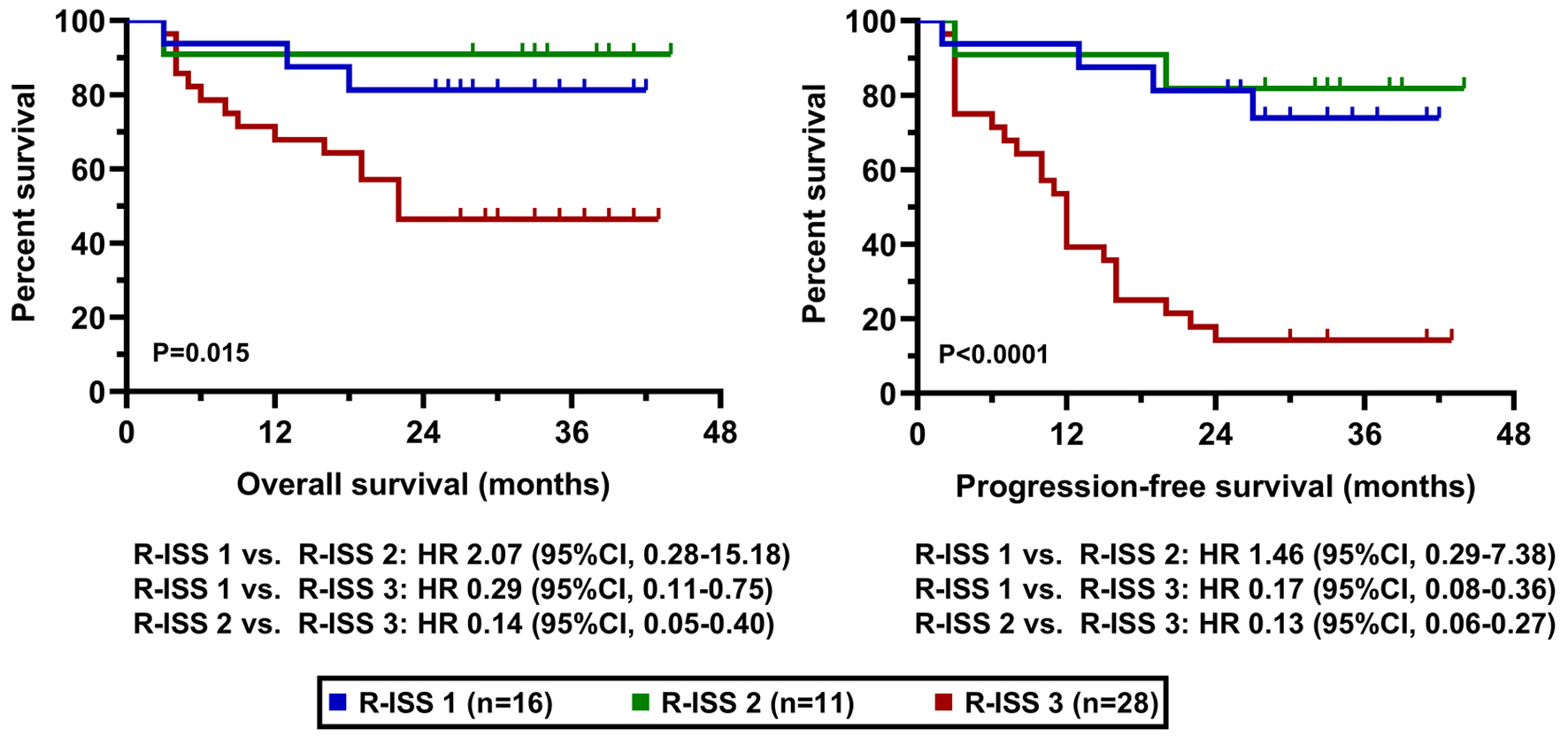 Click for large image | Figure 3. OS and PFS results according to R-ISS. OS: overall survival; PFS: progression-free survival; R-ISS: Revised International Staging System. |
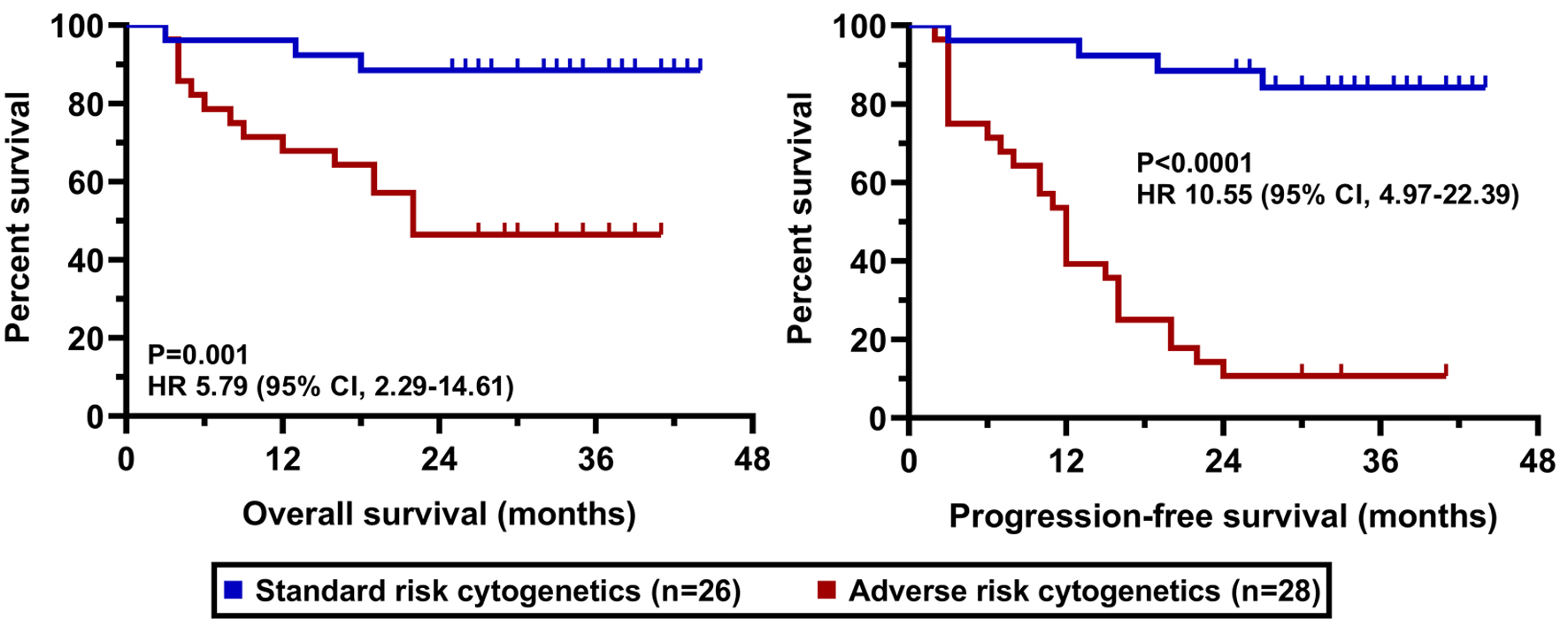 Click for large image | Figure 4. OS and PFS results according to cytogenetic alterations. OS: overall survival; PFS: progression-free survival. |
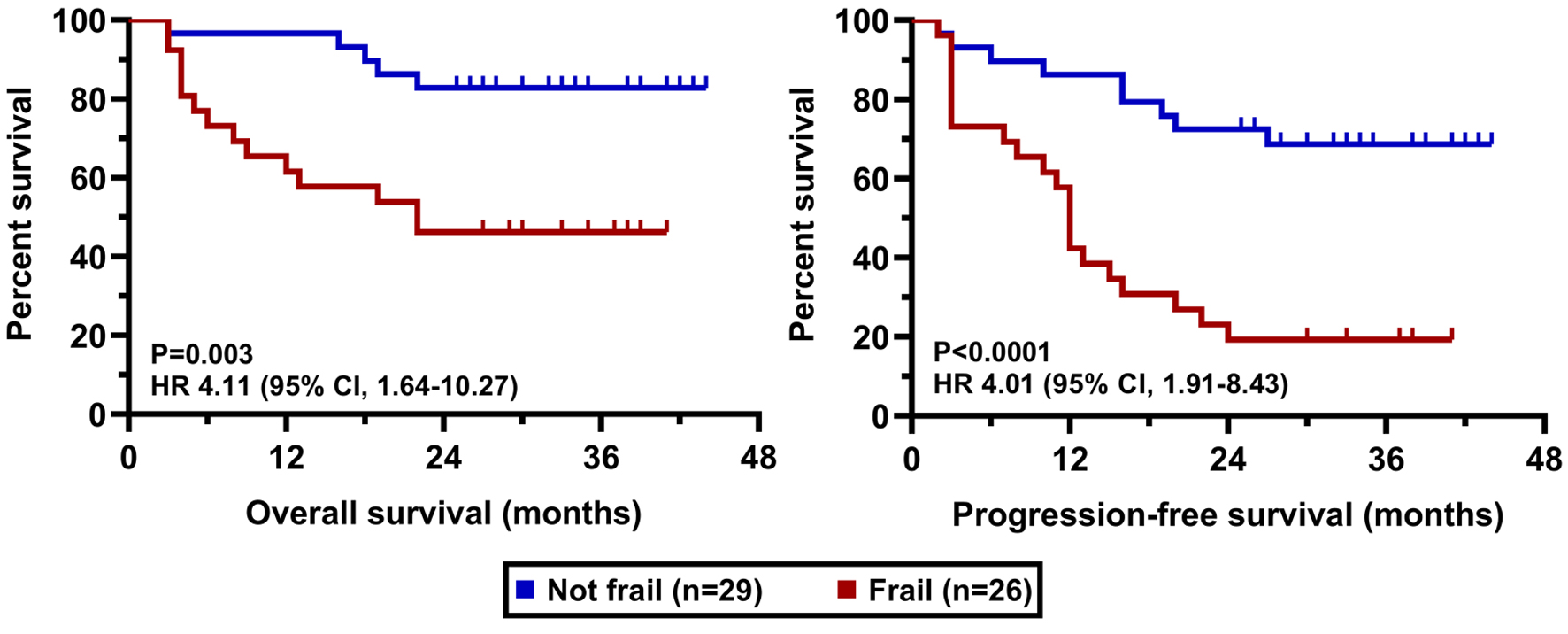 Click for large image | Figure 5. OS and PFS results according to frailty status. OS: overall survival; PFS: progression-free survival. |
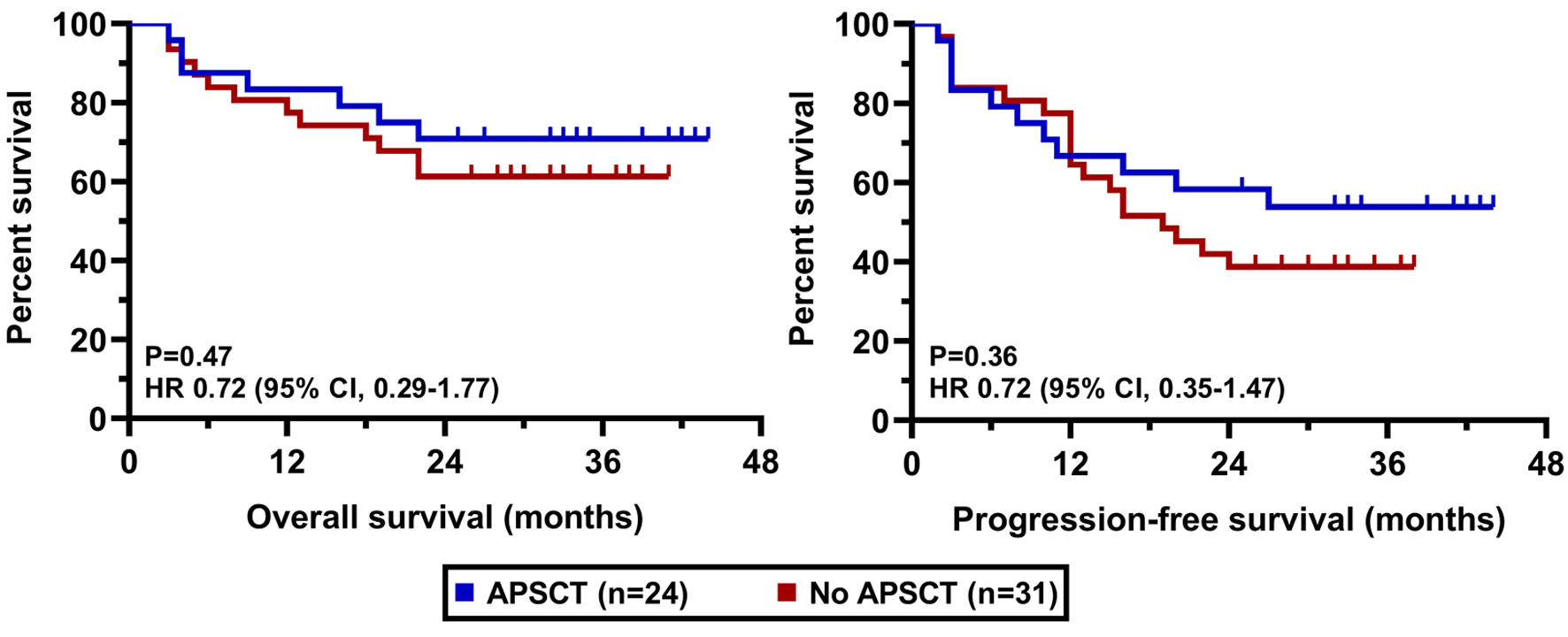 Click for large image | Figure 6. OS and PFS results according to previous APSCT. APSCT: autologous peripheral stem cell transplantation; OS: overall survival; PFS: progression-free survival. |
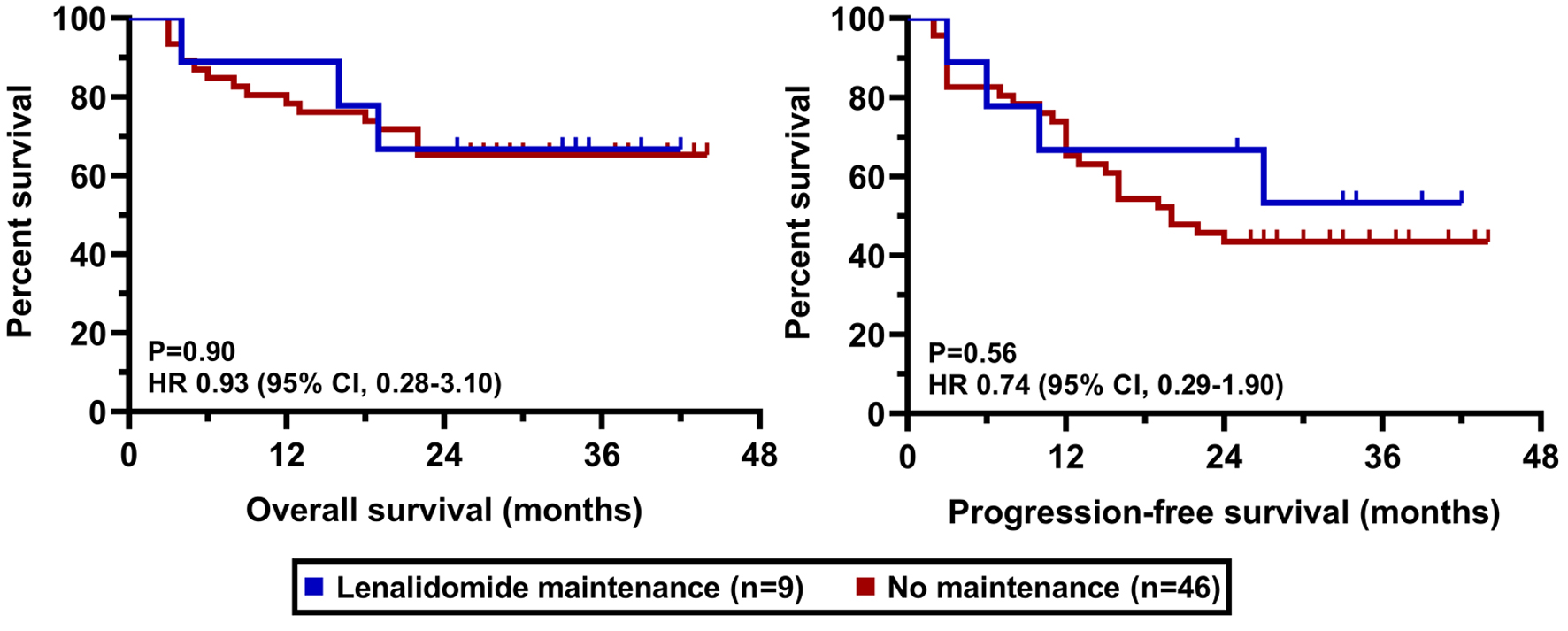 Click for large image | Figure 7. OS and PFS results according to previous lenalidomide exposure. OS: overall survival; PFS: progression-free survival. |
| Discussion | ▴Top |
This retrospective, real-world study evaluated the effectiveness and safety of subcutaneous daratumumab in combination with lenalidomide and dexamethasone (D-Rd) as a second-line therapy with a special reimbursement setting in a cohort of 55 Hungarian patients with RRMM. Our findings demonstrate that D-Rd is a highly effective and well-tolerated regimen in a real-life setting, achieving an ORR of 89.1% and a median PFS of 22.0 months after a median follow-up of 36.6 months. These results are clinically meaningful and contribute valuable evidence to the growing body of real-world data on D-Rd use in early relapse.
In a formerly published article, we investigated the efficacy of daratumumab in heavily pretreated patients (median of three prior lines) and found a median PFS of 17.0 months for the entire cohort, but noted inferior outcomes in the monotherapy and bortezomib-combination groups. We identified that a higher number of prior therapies and advanced ISS stage were key negative prognostic factors [7]. Similarly, the 2019 Japanese study by Kobayashi et al reported a median PFS of 12.4 months in an elderly and heavily pretreated population (median of four prior lines). Crucially, they found that patients with fewer than four prior lines had a significantly higher rate of CR and longer PFS, reinforcing the concept that earlier use of daratumumab yields superior outcomes, a principle our study supports by focusing specifically on the first relapse [8].
The efficacy observed in our recent cohort, while substantial, is lower than the landmark outcomes reported in the pivotal POLLUX trial. In the most recent update of POLLUX with a median follow-up of 79.7 months, the median PFS for patients receiving D-Rd in the second line (one prior therapy) was 53.3 months, and the median OS was 77.8 months [9]. The most plausible explanation lies in the fundamental differences between the highly selected patient populations enrolled in clinical trials and the unselected, heterogeneous populations treated in real-world settings. The POLLUX trial excluded lenalidomide-refractory patients and enrolled subjects with relatively good clinical conditions [10]. Our cohort, while also excluding lenalidomide-refractory patients, likely included a higher proportion of patients with adverse prognostic features and advanced frailty status that might have made them ineligible for such a trial.
This hypothesis is strongly supported by the recent real-world analysis by the Czech Myeloma Group [11]. In their large, multi-center study of 538 RRMM patients, the median PFS for the D-Rd cohort was 23.64 months, a figure remarkably concordant with our finding of 22.0 months. This alignment between two independent real-world analyses from different European countries strongly suggests that a PFS of approximately 23 months may represent the expected effectiveness of D-Rd in broader, unselected clinical practice. Minarik et al’s study further reinforces the impact of high-risk disease, showing that the presence of del(17p) reduced the median PFS for D-Rd to a mere 5.87 months, and high-risk cytogenetics (del(17p), t(4;14), t(14;16)) reduced it to 8.36 months.
Our data directly mirror these findings. We identified that the presence of high-risk cytogenetic alterations and advanced disease stage (R-ISS 3) had a markedly negative impact on patient prognosis. Although the genomic criteria for defining high-risk disease have recently been revised, we continued to regard traditional FISH abnormalities, including t(4;14), t(14;16), and del(17p), as indicators of adverse prognosis [12]. Consequently, a substantial proportion of patients (52.9%) in our cohort exhibited high-risk cytogenetic features. This is a substantially higher proportion than the 13.5-26.7% reported in the large US real-world study by Ailawadhi et al and likely contributes significantly to our inferior survival outcomes. Ailawadhi et al’s study, while concluding that D-Rd improved clinical outcomes overall compared to other Rd-based triplets, also made the critical observation that in patients with high cytogenetic risk all triplet regimens showed comparable effectiveness. This underscores a recurring theme in modern myeloma therapy: high-risk disease biology remains a formidable challenge that even potent combinations like D-Rd cannot fully overcome in a significant portion of patients [13]. Our results, showing a pronounced negative effect of high-risk cytogenetics, are thus consistent with this growing body of evidence.
Another crucial aspect is the evaluation of frailty. In 2015, the International Myeloma Working Group (IMWG) introduced a geriatric assessment (GA)-based frailty score (IMWG frailty score) incorporating age, comorbidities, and functional status, measured by instrumental activities of daily living (IADL) and activities of daily living (ADL), to predict the risk of treatment-related toxicity and mortality among older adults with MM. However, because GA is not routinely implemented in clinical practice due to time and resource limitations, data on the IMWG frailty score are rarely available [14]. More recently, Facon and colleagues proposed a simplified frailty scale as an alternative to the IMWG frailty score, substituting the ECOG PS for physical function (IADL/ADL). Unlike the IMWG frailty score, the simplified scale does not require a formal GA and relies solely on routinely collected clinical variables in oncology practice [15]. Owing to its practicality, the simplified frailty scale has been rapidly adopted by both the myeloma research and clinical communities; therefore, we also employed this method as a pragmatic approach to frailty assessment. The negative impact of frailty on treatment outcomes in MM, as observed in our real-world cohort, is strongly corroborated by the retrospective subgroup analysis of the phase 3 MAIA trial. In this study, the efficacy and safety of daratumumab-lenalidomide-dexamethasone therapy was evaluated in transplant-ineligible newly diagnosed patients. Our findings, demonstrating a markedly negative impact of frail medical conditions on patient prognosis, align with the central conclusion from the MAIA analysis: frail patients consistently experience poorer outcomes compared to their non-frail counterparts. Analyzing the data of patients receiving D-Rd therapy in the MAIA trial, frail individuals had lower PFS rate at 36 months (52.1%) compared to the fit population (78.3%). Although our study involved a relapsed/refractory population treated with D-Rd in the second line, we observed a similar trend where frailty was a key negative prognostic factor. The MAIA analysis also highlighted that frail patients experienced higher rates of grade 3/4 AEs, particularly hematologic toxicities like neutropenia and infections such as pneumonia [16]. This is consistent with our safety data, where the most frequent AEs were hematologic toxicities and infections, including three fatal cases of septicemia.
The presence of frail patients in our real-world cohort, who may receive reduced treatment intensity or experience more dose interruptions, likely contributed to the shorter PFS compared to the robust trial population of POLLUX.
Our finding that neither previous APSCT nor lenalidomide maintenance significantly influenced survival outcomes is intriguing. It may be partly explained by the overpowering negative prognostic impact of high-risk cytogenetics and frailty in our cohort. It could also suggest that the efficacy of D-Rd is substantial enough to partially overcome the potential negative influence of prior lenalidomide exposure (in non-refractory patients), a finding that was also seen in the POLLUX subgroup analysis [17].
When comparing our response rates, the ORR of 89% (with 22% CR, 40% VGPR, 27% PR) is respectable and aligns more closely with the 92.9% ORR from POLLUX and the 91.4% from the findings of the Czech Myeloma Group [6, 11]. This indicates that the D-Rd regimen is highly active in inducing initial responses even in a real-world population. The disparity between a high ORR and a shorter PFS suggests that the primary challenge in our cohort is not in achieving a response, but in maintaining it. This could be due to the high-risk disease biology leading to early relapse, or potentially to difficulties in maintaining the planned treatment intensity over the long term due to cumulative toxicities, patient compliance issues, or logistical challenges in the healthcare system- factors absent in the controlled environment of a clinical trial [18].
The treatment patterns observed in our study, where D-Rd was successfully deployed in the second line following a special reimbursement initiative in Hungary, reflect a global trend towards the earlier use of daratumumab-based combinations. The German study by Steinmetz et al on physician decision-making provides a relevant context. In their real-world analysis, D-Rd was the most frequently prescribed second-line regimen (16.5%), with physicians citing “treatment effectiveness” as the primary reason for selection. This mirrors the clinical rationale in our study and confirms that the efficacy profile of D-Rd established in clinical trials is the driving force behind its adoption in routine practice. The German study also noted that the goal of inducing the “deepest possible response” was a key driver for choosing triplet regimens like D-Rd, an objective supported by the high response rates (89.1%) and the 21.8% CR rate we observed [19].
Regarding safety, our data corroborate the well-established tolerability profile of subcutaneous daratumumab. The most frequent AEs were mild injection-related reactions and manageable hematologic toxicities, predominantly neutropenia. This is consistent with the long-term safety data from POLLUX, where the most common grade 3/4 event was neutropenia (57.6%), and the regimen did not lead to an unacceptable rate of treatment discontinuation [20]. However, we have to underline again that the incidence of AEs was significantly higher among our frail patients compared with non-frail patients. Improved infection control and early intervention may further reduce the incidence of such complications. Otherwise, the absence of new safety signals in our real-world cohort is reassuring for clinicians implementing this therapy in broader patient populations.
Several limitations of our study must be acknowledged. Its retrospective nature and relatively small sample size from a single country limit the generalizability of the findings. The lack of a control group treated with an alternative regimen prevents direct comparative effectiveness conclusions within our cohort. Furthermore, the median follow-up time was insufficient to report mature OS data, a key efficacy endpoint. Finally, data on minimal residual disease, a critical prognostic marker strongly associated with superior OS in the POLLUX trial, were not available in our retrospective analysis [21].
Conclusion
In conclusion, our real-world analysis confirms that D-Rd is an effective and safe second-line therapy for RRMM. The median PFS of 22.0 months, while inferior to the landmark results of the POLLUX clinical trial, is highly consistent with other contemporary real-world studies. The primary explanation for this discrepancy is the markedly different patient population, characterized by a high prevalence of adverse prognostic features such as high-risk cytogenetics and frailty, which are underrepresented in clinical trials but form a substantial part of routine clinical practice (Table 3). Our study therefore provides a crucial and realistic expectation for clinicians utilizing this potent regimen. It underscores the persistent and unmet need for more effective strategies to overcome the poor prognosis associated with high-risk MM, even in the era of monoclonal antibodies. As the treatment landscape continues to evolve with the introduction of bispecific antibodies and CAR-T therapies, establishing the real-world effectiveness of current standards of care remains essential for optimizing patient management and guiding future therapeutic sequences [22].
 Click to view | Table 3. Comparison of Baseline Patient Characteristics Across Clinical Trials Evaluating the Efficacy of D-Rd Therapy in Relapsed or Refractory Multiple Myeloma |
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None of the authors had any conflict of interest.
Informed Consent
No informed consent was required for retrospective data collection.
Author Contributions
Conceptualization, formal analysis, data curation, visualization, and writing - original draft preparation: Szilvia Lovas, Nora Obajed Al-Ali, Katalin Farkas, and Laszlo Varoczy; statistical analysis: Laszlo Imre Pinczes; treatment of the patients, investigation, and resources: Gergely Varga, Gabor Mikala, Peter Rajnics, Szabolcs Kosztolanyi, Mark Plander, Piroska Pettendi, and Laszlo Varoczy; supervision: Laszlo Varoczy; writing - review and editing: Szilvia Lovas, Nora Obajed Al-Ali, Katalin Farkas, Gergely Varga, Gabor Mikala, and Laszlo Varoczy. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary files.
Abbreviations
ADCC: antibody-dependent cellular cytotoxicity; ADL: activities of daily living; AE: adverse event; APSCT: autologous peripheral stem cell transplantation; CAR-T: chimeric antigen receptor T-cell; CD: cluster designation; CDC: complement-dependent cytotoxicity; CR: complete response; del: deletion; D-Rd: daratumumab, lenalidomide, and dexamethasone; ECOG PS: Eastern Cooperative Oncology Group Performance Status; FISH: fluorescence in situ hybridization; GA: geriatric assessment; GFR: glomerular filtration rate; IADL: instrumental activities of daily living; Ig: immunoglobulin; IMWG: International Myeloma Working Group; MM: multiple myeloma; MRD: minimal residual disease; ORR: overall response rate; OS: overall survival; PD: progressive disease; PFS: progression-free survival; PR: partial response; Rd: lenalidomide and dexamethasone; R-ISS: Revised International Staging System; RRMM: relapsed or refractory multiple myeloma; SD: stable disease; t: translocation; VGPR: very good partial response
| References | ▴Top |
- Cowan AJ, Green DJ, Kwok M, Lee S, Coffey DG, Holmberg LA, Tuazon S, et al. Diagnosis and management of multiple myeloma: a review. JAMA. 2022;327(5):464-477.
doi pubmed - Dimopoulos MA, Terpos E, Boccadoro M, Moreau P, Mateos MV, Zweegman S, Cook G, et al. EHA-EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma. Nat Rev Clin Oncol. 2025;22(9):680-700.
doi pubmed - de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186(3):1840-1848.
doi pubmed - van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, Lokhorst HM, et al. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 2011;96(2):284-290.
doi pubmed - Arnall JR, Maples KT, Harvey RD, Moore DC. Daratumumab for the treatment of multiple myeloma: a review of clinical applicability and operational considerations. Ann Pharmacother. 2022;56(8):927-940.
doi pubmed - Bahlis NJ, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M, Ho PJ, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34(7):1875-1884.
doi pubmed - Lovas S, Varga G, Farkas P, Masszi T, Wohner N, Bereczki A, Adamkovich N, et al. Real-world data on the efficacy and safety of daratumumab treatment in Hungarian relapsed/refractory multiple myeloma patients. Int J Hematol. 2019;110(5):559-565.
doi pubmed - Kobayashi H, Tsushima T, Terao T, Abe Y, Miura D, Narita K, Kitadate A, et al. Evaluation of the safety and efficacy of daratumumab outside of clinical trials. Int J Hematol. 2019;109(6):665-672.
doi pubmed - Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, et al. Overall survival with daratumumab, lenalidomide, and dexamethasone in previously treated multiple myeloma (POLLUX): a randomized, open-label, phase III trial. J Clin Oncol. 2023;41(8):1590-1599.
doi pubmed - Dimopoulos MA, San-Miguel J, Belch A, White D, Benboubker L, Cook G, Leiba M, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica. 2018;103(12):2088-2096.
doi pubmed - Minarik J, Pour L, Latal V, Jelinek T, Straub J, Jungova A, Radocha J, et al. Lenalidomide based triplets in relapsed/refractory multiple myeloma: analysis of the Czech Myeloma Group. BMC Cancer. 2025;25(1):651.
doi pubmed - Avet-Loiseau H, Davies FE, Samur MK, Corre J, D'Agostino M, Kaiser MF, Raab MS, et al. International myeloma society/international myeloma working group consensus recommendations on the definition of high-risk multiple myeloma. J Clin Oncol. 2025;43(24):2739-2751.
doi pubmed - Ailawadhi S, Cheng M, Cherepanov D, DerSarkissian M, Stull DM, Hilts A, Chun J, et al. Comparative effectiveness of lenalidomide/dexamethasone-based triplet regimens for treatment of relapsed and/or refractory multiple myeloma in the United States: An analysis of real-world electronic health records data. Curr Probl Cancer. 2024;50:101078.
doi pubmed - Gahagan A, Maheshwari S, Rangarajan S, Ubersax C, Tucker A, Harmon C, Pasala MS, et al. Evaluating concordance between International Myeloma Working Group (IMWG) frailty score and simplified frailty scale among older adults with multiple myeloma. J Geriatr Oncol. 2024;15(8):102051.
doi pubmed - Facon T, Dimopoulos MA, Meuleman N, Belch A, Mohty M, Chen WM, Kim K, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34(1):224-233.
doi pubmed - Facon T, Cook G, Usmani SZ, Hulin C, Kumar S, Plesner T, Touzeau C, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077.
doi pubmed - Kaufman JL, Dimopoulos MA, White D, Benboubker L, Cook G, Leiba M, Morton J, et al. Daratumumab, lenalidomide, and dexamethasone in relapsed/refractory myeloma: a cytogenetic subgroup analysis of POLLUX. Blood Cancer J. 2020;10(11):111.
doi pubmed - Flowers CR, Anantha RW, Leautaud V, Desai P, Donald CE, Hildebrandt MAT, Koff JL, et al. Addressing health disparities in hematologic malignancies: from genes to outreach. Blood Cancer Discov. 2025;6(2):79-93.
doi pubmed - Steinmetz HT, Ertel F, Brinkmann B, Houghton K, Nham T, Flossmann C. Treatment patterns, goals, and decision-making criteria for second- and third-line therapies for multiple myeloma in Germany. Adv Ther. 2025;42(8):4023-4047.
doi pubmed - Mateos MV, Spencer A, Nooka AK, Pour L, Weisel K, Cavo M, Laubach JP, et al. Daratumumab-based regimens are highly effective and well tolerated in relapsed or refractory multiple myeloma regardless of patient age: subgroup analysis of the phase 3 CASTOR and POLLUX studies. Haematologica. 2020;105(2):468-477.
doi pubmed - Cavo M, San-Miguel J, Usmani SZ, Weisel K, Dimopoulos MA, Avet-Loiseau H, Paiva B, et al. Prognostic value of minimal residual disease negativity in myeloma: combined analysis of POLLUX, CASTOR, ALCYONE, and MAIA. Blood. 2022;139(6):835-844.
doi pubmed - Zhu L, Nawaz MA, Zuo Y, Zeng P. Next-generation immunotherapy in relapsed/refractory multiple myeloma: Strategies to achieve sustained MRD negativity. Crit Rev Oncol Hematol. 2025;214:104913.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.