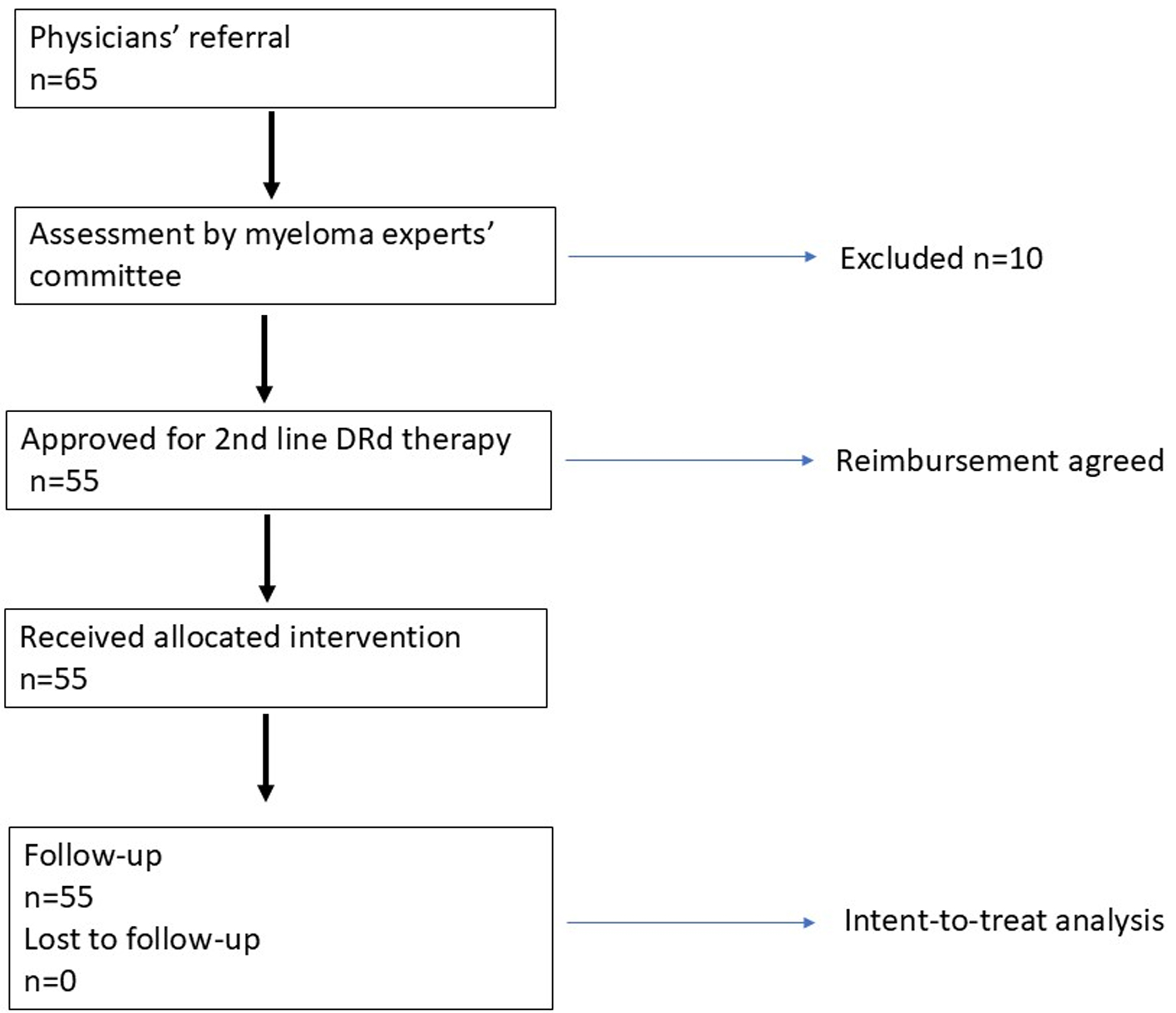
Figure 1. Study design.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 6, December 2025, pages 314-323
Efficacy and Safety of Daratumumab, Lenalidomide and Dexamethasone Therapy in the First Relapse of Multiple Myeloma Patients – Real World Data from Hungary
Figures

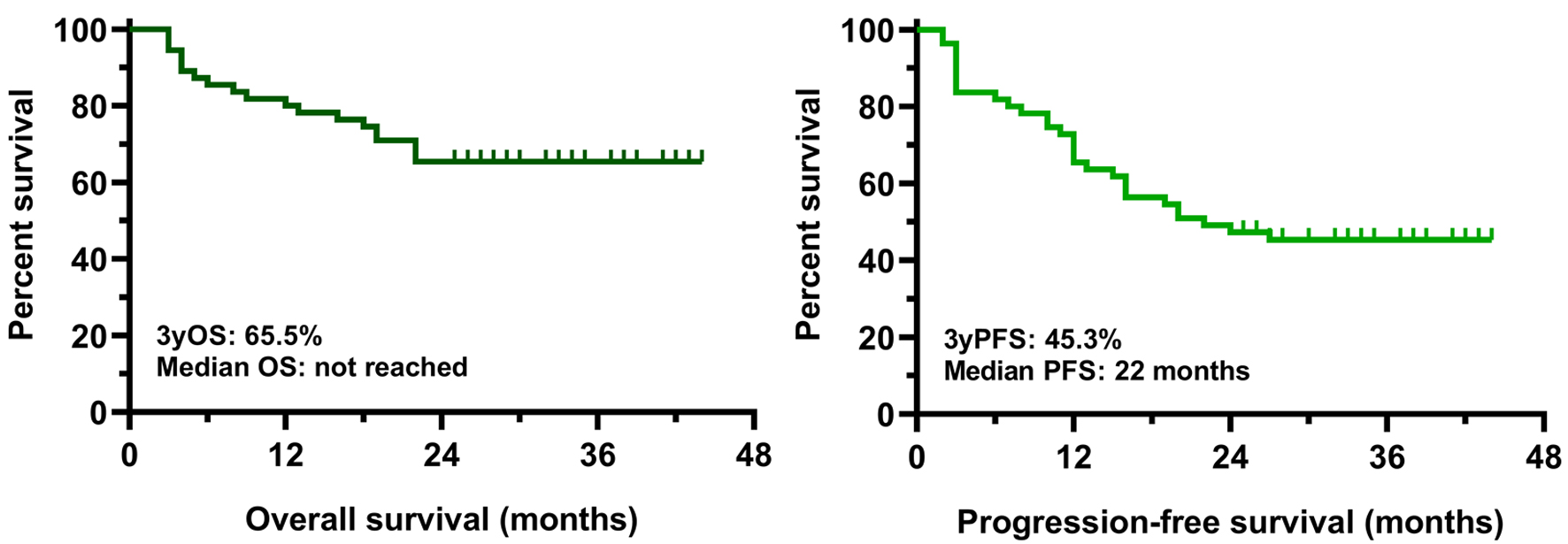
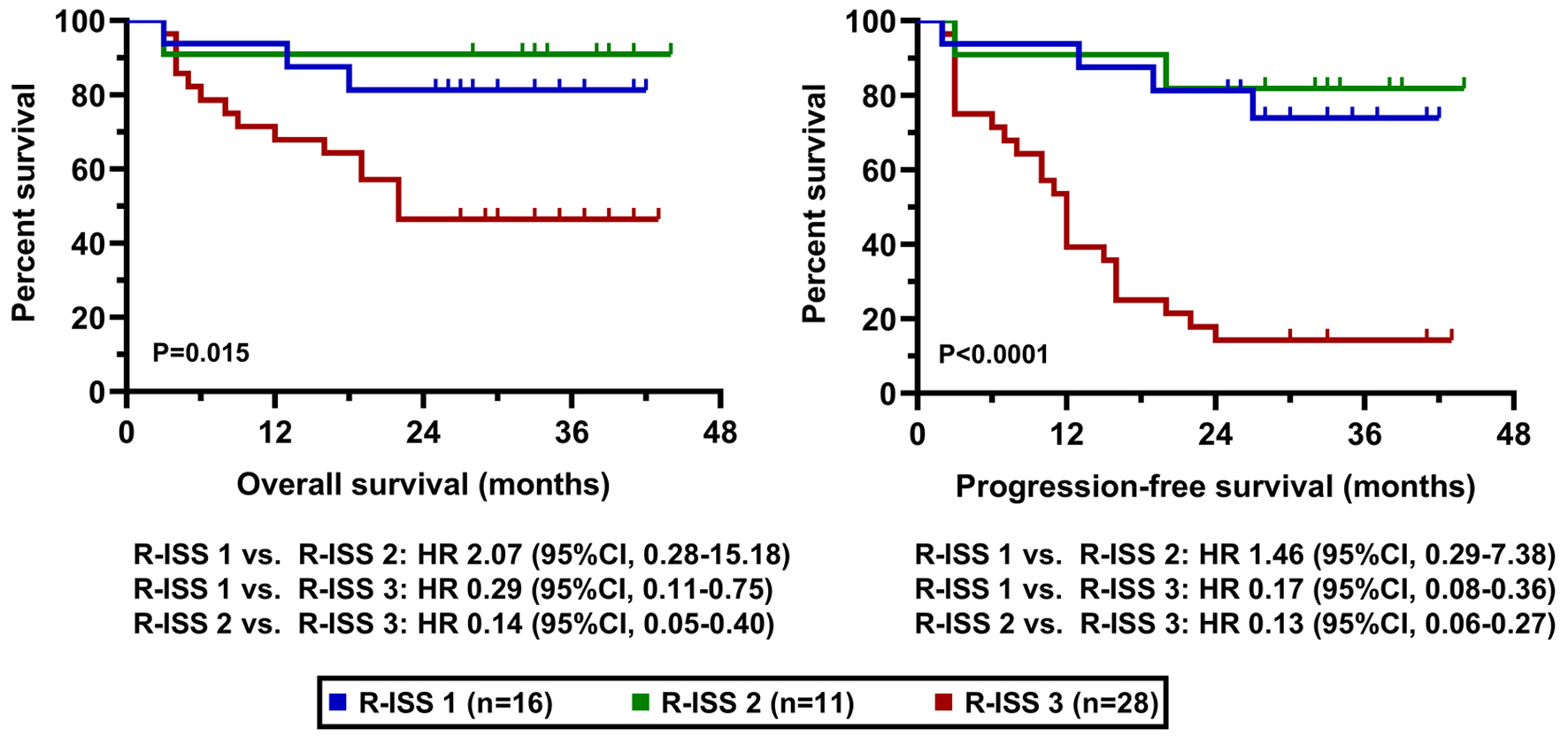
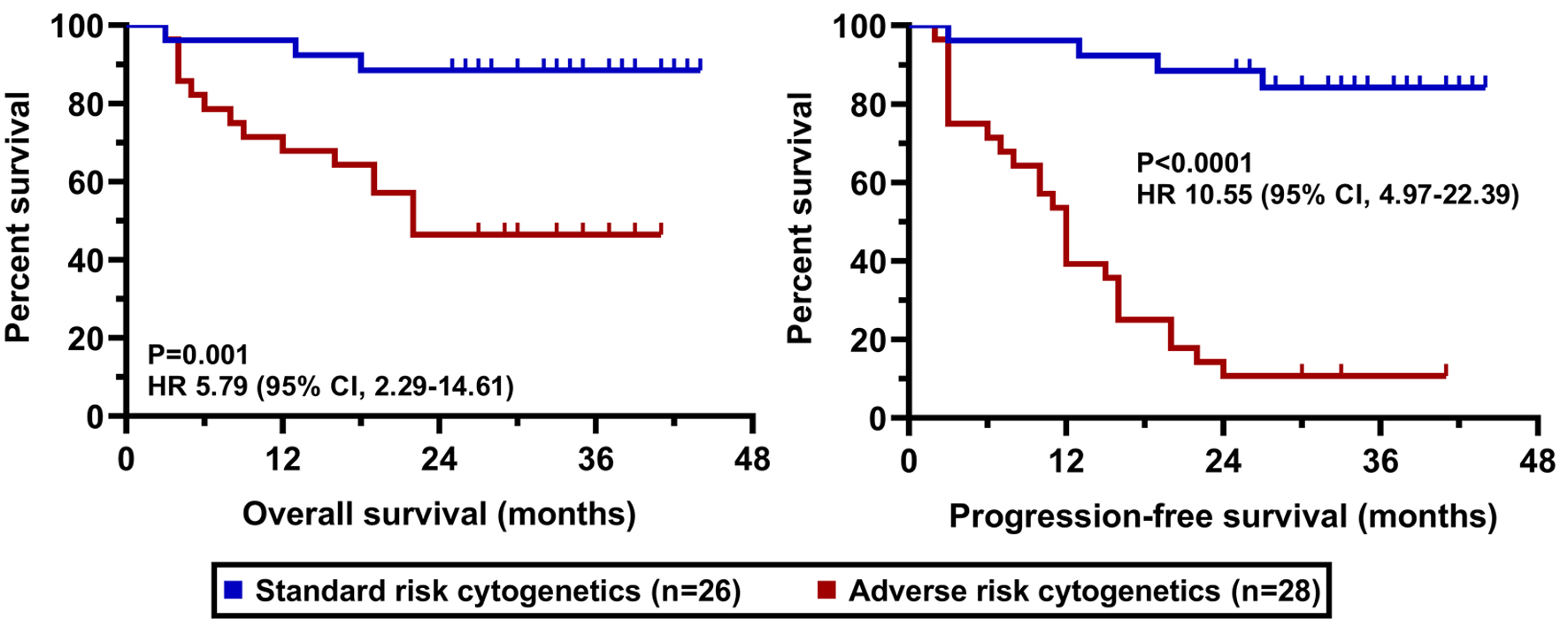
Tables
| Characteristic | |
|---|---|
| APSCT: autologous peripheral stem cell transplantation; D-Rd: daratumumab, lenalidomide, and dexamethasone; Ig: immunoglobulin; R-ISS: Revised International Staging System. | |
| Age at treatment onset (years), median (range) | 65 (41 - 78) |
| Gender, n (%) | |
| Male | 26 (47) |
| Female | 29 (53) |
| Frailty status, n (%) | |
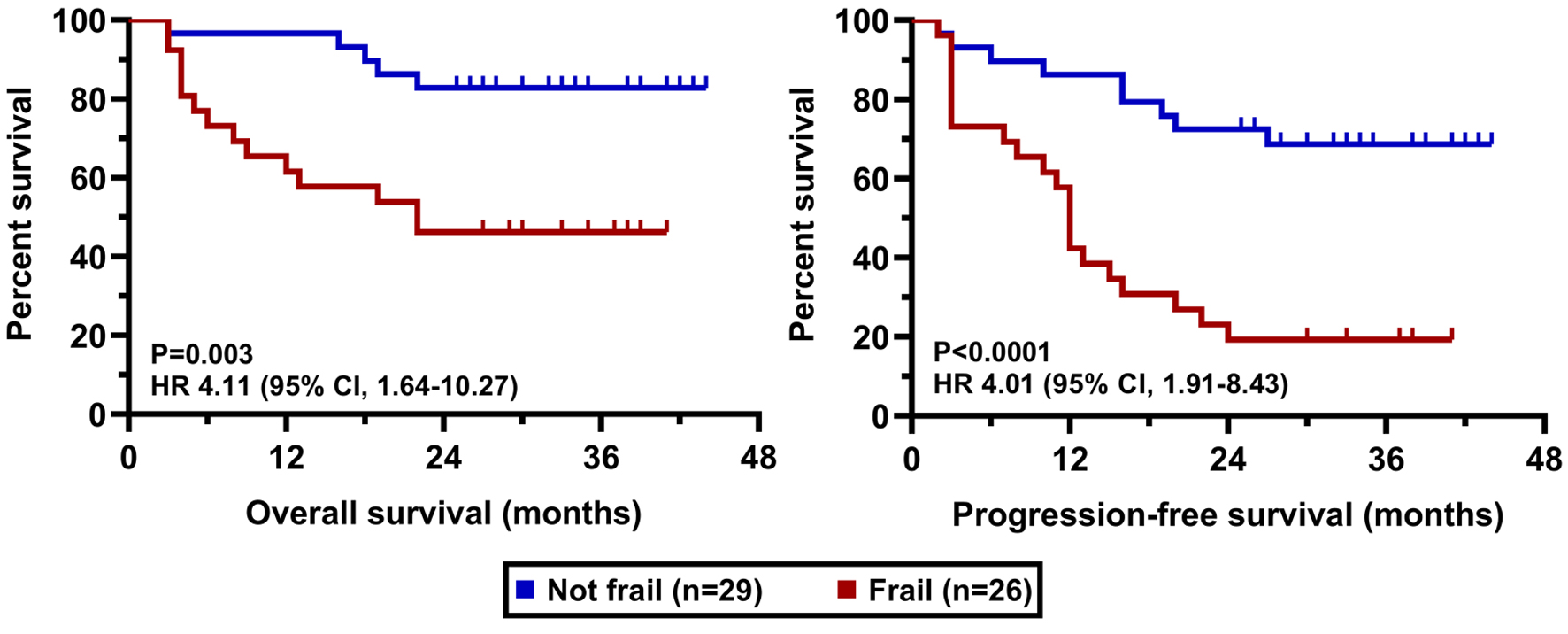
| Frail | 26 (47) |
| Non-frail | 29 (53) |
| Type of measurable disease, n (%) | |
| IgG | 29 (52.7) |
| IgA | 14 (25.4) |
| Light-chain | 12 (21.9) |
| R-ISS disease staging, n (%) | |
| I | 16 (29) |
| II | 11 (20) |
| III | 28 (51) |
| Prior treatments, n (%) | |
| Bortezomib | 54(98) |
| Thalidomide | 17 (31) |
| Alkylating agent | 24 (44) |
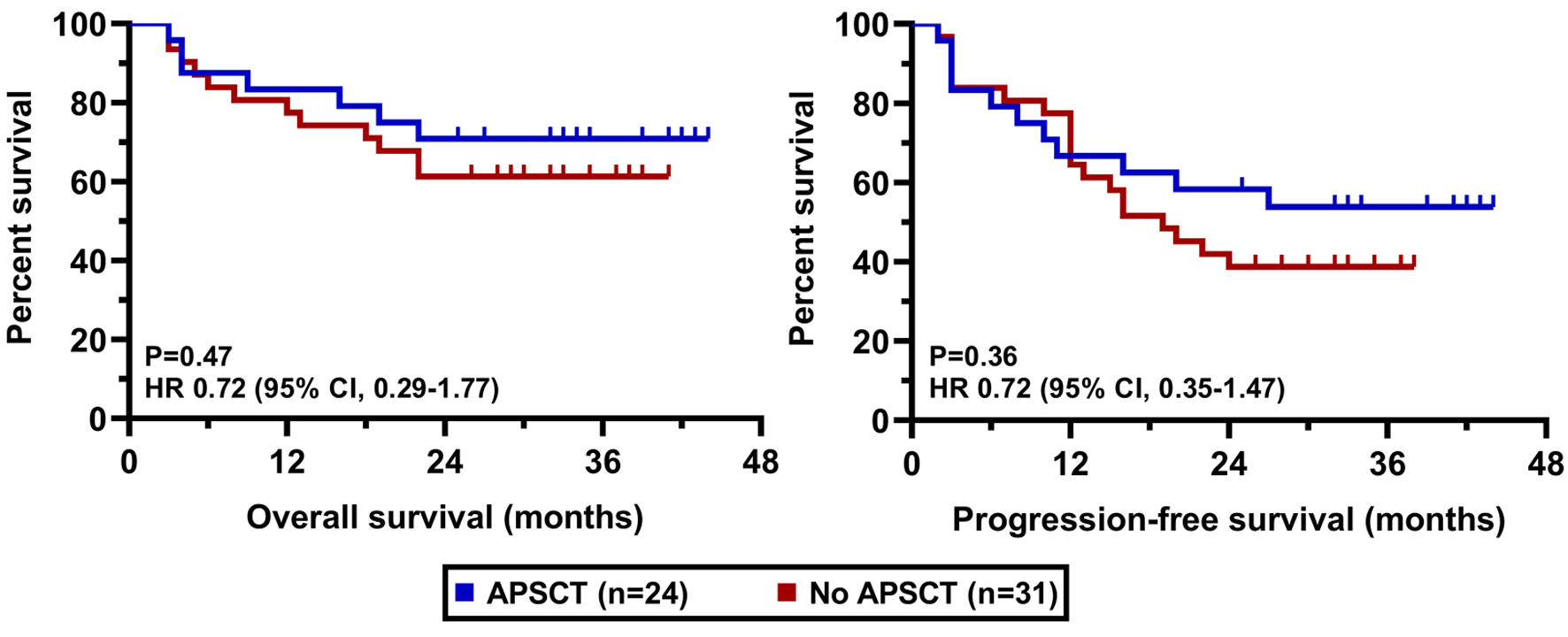
| APSCT | 23 (43) |
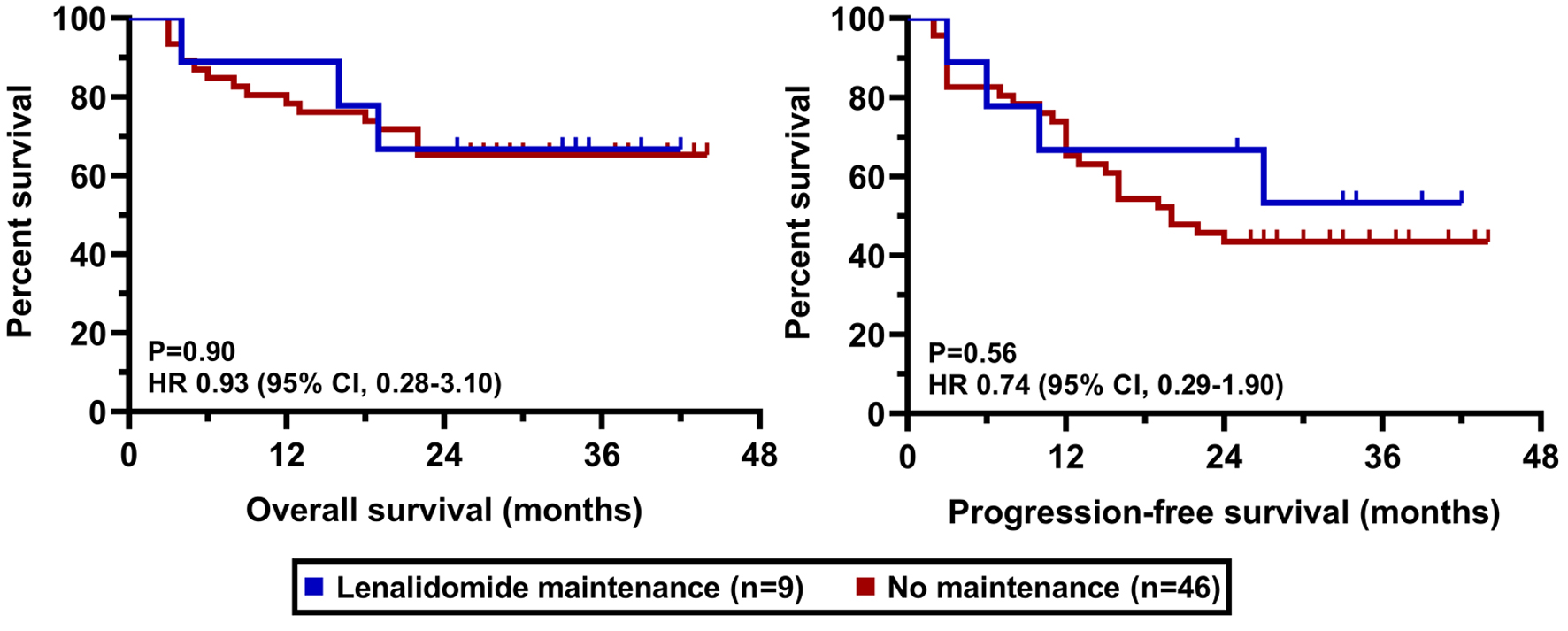
| Lenalidomide maintenance | 9 (16) |
| Pre-D-Rd cytogenetic profile, n (%) | |
| Standard risk cytogenetic abnormality | 25/53 (47.1)) |
| High risk cytogenetic abnormality | 28/53 (52.9) |
| Grade 1 - 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|
| Neutropenia | 25 | 4 | ||
| Thrombocytopenia | 18 | 3 | ||
| Anemia | 16 | 1 | ||
| Infections | 15 | 1 | 1 | 3 |
| Gastrointestinal | 20 | |||
| Infusion-related | 32 | |||
| All | 126 | 9 | 1 | 3 |
| POLLUX trial | Czech Myeloma Group data | US real-world data | Hungarian National trial | |
|---|---|---|---|---|
| aR-ISS staging system was used. D-Rd: daratumumab, lenalidomide, and dexamethasone; R-ISS: Revised International Staging System. | ||||
| Number of patients | 286 | 224 | 214 | 55 |
| Previous treatment lines | 1 - 9 | 1 - 3 | 1 - 4+ | 1 |
| Age | 65 (34 - 89) | 65.5 (45.0 - 77.8) | 68.6 (41.2 - 83.4) | 65 (41 - 78) |
| ISS stages, n (%) | ||||
| 1 | 137 (48) | 89 (48.4) | 50 (23.4) | 16 (29) |
| 2 | 93 (32) | 41 (22.3) | 42 (19.6) | 11 (20) |
| 3 | 56 (20) | 54 (29.3) | 46 (21.5) | 28 (51)a |
| Unknown | 40 (17.8) | 76 (35.5) | ||
| Cytogenetic alterations, n (%) | Not reported | |||
| Standard | 193 (67) | 163 (76.2) | 25 (45) | |
| High-risk | 35 (12) | 32 (15) | 28 (51) | |
| Unknown | 58 (21) | 19 (8.9) | 2 (4) | |
| Frailty status, n (%) | Not reported | Not reported | ||
| Frail | 35 (16.4) | 26 (47) | ||
| Non-frail | 179 (83.6) | 29 (53) | ||