| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Review
Volume 14, Number 4, August 2025, pages 193-201
B-Cell Lymphoma 2 Inhibition in Acute Lymphoblastic Leukemia: Mechanisms, Resistance, and Emerging Combinations With Venetoclax
Amr Hanbalia, b , Ahmed Kotba, Mostafa Saleha
aKing Faisal Specialist Hospital & Research Center, Riyadh, KSA
bCorresponding Author: Amr Hanbali, King Faisal Specialist Hospital & Research Center, Riyadh 11211, KSA
Manuscript submitted June 2, 2025, accepted August 5, 2025, published online August 25, 2025
Short title: Venetoclax Combinations in ALL
doi: https://doi.org/10.14740/jh2092
- Abstract
- Introduction
- Role of B-Cell Lymphoma 2 (BCL-2) in ALL
- Introduction to Venetoclax
- Preclinical Evidence Supporting Venetoclax in ALL
- Clinical Studies of Venetoclax-Based Combinations in ALL
- Mechanisms of Resistance and Strategies to Overcome Them
- Safety and Tolerability
- Future Directions and Ongoing Trials
- Conclusions
- References
| Abstract | ▴Top |
Recent studies show that the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax is a promising therapeutic drug for acute lymphoblastic leukemia (ALL), especially in high-risk subtypes including early T-cell precursor (ETP)-ALL, Philadelphia chromosome (Ph)-like B-cell ALL, and KMT2A-rearranged leukemia. The preclinical and early-phase clinical research shows that venetoclax-based combinations can increase apoptosis and improve response rates when used with chemotherapy or hypomethylating agents. The main challenge to venetoclax efficacy remains the resistance mechanisms that primarily involve myeloid cell leukemia-1 (MCL-1) and BCL-extra large (XL). This review provides an overview of the current state of venetoclax in ALL by discussing its mechanistic basis and clinical trial results as well as safety data and strategies to overcome resistance and enhance venetoclax-based treatments.
Keywords: Venetoclax; BCL-2 inhibition; Acute lymphoblastic leukemia; T-cell ALL; B-cell ALL; Apoptosis resistance; Combination therapy; Targeted therapy
| Introduction | ▴Top |
Acute lymphoblastic leukemia (ALL) represents a diverse group of hematologic malignancies which develops from immature lymphoid precursors that multiply in bone marrow and peripheral blood and other tissues. The two main types of ALL are B-cell ALL (B-ALL) and T-cell ALL (T-ALL) which differ by the cell lineage of leukemic blasts. B-ALL represents 75-85% of pediatric cases and 60-70% of adult cases [1]. The different subtypes of leukemia contain multiple genetic and molecular abnormalities which include BCR-ABL1 in Philadelphia chromosome (Ph)+ ALL, KMT2A rearrangements and ETV6-RUNX1 among others as well as copy number variations and somatic mutations that affect both prognosis and treatment response [2, 3].
T-ALL presents specific challenges because it often develops from early T-cell precursor (ETP) cells, contains high-risk genetic abnormalities, and shows resistance to standard chemotherapy treatments [4]. B-ALL contains different subtypes which produce distinct treatment outcomes. Patients with hyperdiploid or ETV6-RUNX1-positive B-ALL have better prognoses, while patients with Ph-like or KMT2A-rearranged B-ALL experience worse survival and higher relapse rates [3, 5].
Risk-adapted therapy has improved survival rates for pediatric patients but relapsed or refractory (R/R) ALL remains a major clinical challenge with unfavorable prognoses. The current standard salvage treatments produce minimal long-lasting responses while patients with multiple relapses or those who cannot receive allogeneic hematopoietic cell transplantation (allo-HCT) have few available treatment options. The molecular complexity and clonal evolution of ALL at relapse contribute to therapeutic resistance [6], underscoring the urgent need for novel targeted therapies that can overcome these barriers and improve long-term outcomes across ALL subtypes.
| Role of B-Cell Lymphoma 2 (BCL-2) in ALL | ▴Top |
The process of leukemogenesis and treatment resistance in ALL depends on the dysregulation of apoptosis. The BCL-2 family proteins function as key regulators to control the process of mitochondrial outer membrane permeabilization and subsequent caspase activation. The therapeutic evasion occurs because of the overexpression of anti-apoptotic members BCL-2, myeloid cell leukemia-1 (MCL-1), and BCL-extra large (XL) [7, 8] (Fig. 1a).
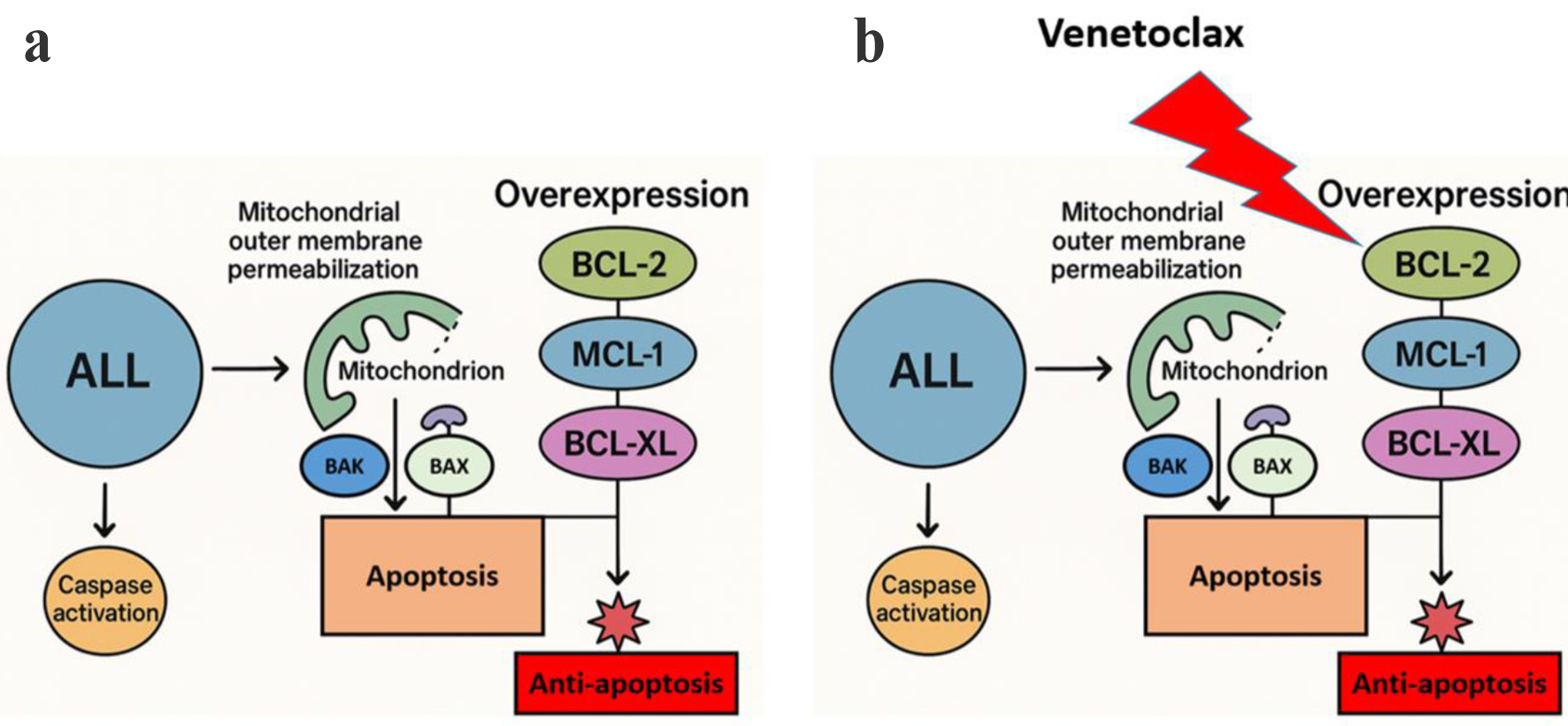 Click for large image | Figure 1. Mechanism of venetoclax-induced apoptosis in ALL. (a) ALL cells express elevated levels of anti-apoptotic BCL-2 family proteins (BCL-2, MCL-1, and BCL-XL) which prevent mitochondrial outer membrane permeabilization (MOMP) and trap pro-apoptotic proteins (BAX and BAK) to stop caspase activation and apoptosis. (b) Venetoclax specifically targets BCL-2 to enable apoptotic signaling through MOMP and caspase activation. The compensatory increase of MCL-1 and BCL-XL proteins helps to preserve anti-apoptotic signaling which leads to venetoclax resistance. ALL: acute lymphoblastic leukemia; BCL-2: B-cell lymphoma 2; MCL-1: myeloid cell leukemia-1; XL: extra large. |
The expression levels of BCL-2 differ between different ALL subtypes. The research by Coustan-Smith et al demonstrated that B-lineage ALL blasts showed significant elevation of BCL-2 expression compared to normal B-cell progenitors while surviving better in cytokine-deprived conditions. The study by Coustan-Smith et al found that while BCL-2 expression was elevated in B-lineage ALL blasts compared to normal B-cell progenitors, its expression was relatively lower in leukemias with adverse cytogenetic abnormalities such as the Ph, suggesting that BCL-2 overexpression is more common in cytogenetically favorable cases [9]. These findings indicate that elevated BCL-2 expression confers a survival advantage to leukemic blasts by enhancing resistance to apoptosis in cytokine-deprived conditions; however, this does not universally correlate with poor clinical outcomes or treatment resistance.
The study by Tzifi et al provided extensive evidence about BCL-2 family members in leukemia through their description of how BCL-2, MCL-1, BAX, and BH3-only proteins BID and NOXA work together in a delicate balance. The network’s dysregulation leads to disease progression through impaired apoptosis especially when BCL-2 becomes abnormally overexpressed [10].
| Introduction to Venetoclax | ▴Top |
The potent oral drug venetoclax functions as a small-molecule inhibitor which specifically targets anti-apoptotic protein (BCL-2), to regulate the intrinsic mitochondrial apoptosis pathway (Fig. 1b). The mechanism of venetoclax mimics BH3-only protein activity to remove BIM and other pro-apoptotic molecules from BCL-2 which enables apoptosis in cancer cells that rely on BCL-2 for survival. The US FDA has approved venetoclax for chronic lymphocytic leukemia (CLL) patients with 17p deletion and for elderly or unfit acute myelocytic leukemia (AML) patients who receive it with hypomethylating agents (HMAs) or low-dose cytarabine, thus transforming treatment approaches for hematologic malignancies [11, 12]. The increasing interest in using venetoclax for ALL treatment stems from BCL-2 overexpression in specific ALL subgroups including early precursor B-ALL and myeloid-lymphoid leukemia (MLL)/KMT2A rearrangement cases. The therapeutic potential of venetoclax in ALL models has been demonstrated through preclinical studies, yet its clinical effectiveness remains limited because MCL-1 and BCL-XL expression compensates for its effects, thus requiring the development of rational combination strategies to enhance its clinical application in this context [7, 9].
| Preclinical Evidence Supporting Venetoclax in ALL | ▴Top |
Research conducted before clinical trials demonstrated that specific cases of ALL including ETP-ALL and high-risk B-ALL depend on BCL-2 survival mechanisms which makes them vulnerable to venetoclax treatment. ETP-ALL represents a distinct biological T-ALL subtype which shows high BCL-2 expression levels together with low BIM and BAX pro-apoptotic protein amounts, thus creating a therapeutic opportunity [13]. Xenograft and cell line experiments demonstrated that venetoclax specifically kills ETP-ALL blasts because of their apoptotic weakness [14]. The B-ALL subtypes that have MLL/KMT2A rearrangements or early progenitor immunophenotypes express elevated BCL-2 levels and show sensitivity to venetoclax treatment in both laboratory and animal studies [9].
Venetoclax demonstrates enhanced antileukemic effects when used in combination with other cytotoxic drugs. The combination of homoharringtonine (a translation inhibitor) with venetoclax led to substantial apoptosis in B-ALL models through MCL-1 downregulation which acts as a main resistance factor to venetoclax [15]. The combination of venetoclax with asparaginase produced enhanced apoptosis through dual mechanisms where venetoclax blocked BCL-2 while asparaginase reduced MCL-1 by depleting glutamine and asparagine which are essential for MCL-1 translation and stability [16]. These findings supported using venetoclax as part of multi-agent clinical trial regimens especially for treating high-risk or R/R ALL patients.
| Clinical Studies of Venetoclax-Based Combinations in ALL | ▴Top |
Earlier studies
The first clinical evidence of venetoclax effectiveness in T-ALL and ETP-ALL patients emerged from two elderly patients who achieved morphologic remission while one patient became minimal residual disease (MRD) negative but the other patient relapsed after an initial response [14]. A phase I study was designed subsequently to evaluate venetoclax at 600 mg per day in combination with mini-hyper-CVD (dose-reduced hyperfractionated cyclophosphamide, vincristine, dexamethasone, alternating with methotrexate and cytarabine; anthracycline omitted) for older adults who had untreated or R/R Ph-negative ALL. The study involved 10 untreated patients with a median age of 65 who achieved a complete remission (CR) rate of 90% and all patients became MRD negative with no relapses during 11.3 months of follow-up; the R/R group achieved a complete remission or complete remission with incomplete platelet recovery (CR/CRp) rate of 37.5% with tolerable side effects [17]. The phase II trial used venetoclax in combination with mini-hyper-CVD to treat 23 patients who were either newly diagnosed or had R/R disease. The study produced CR rates of 75% in frontline patients and 65% in evaluable R/R patients who had adverse cytogenetics and previous allo-HCT or immunotherapy experience. The study demonstrated both long-lasting remission and successful transplant preparation [18]. The retrospective study evaluated 13 R/R T-ALL patients who achieved 60% marrow remission and 30% full hematologic recovery despite their high-risk features and previous treatments. The treatment involved administering venetoclax at 200 mg/day for 21 days together with hyper-CVAD (a hyperfractionated chemotherapy regimen alternating cyclophosphamide, vincristine, doxorubicin, and dexamethasone with high-dose methotrexate and cytarabine) or nelarabine; the median overall survival (OS) and RFS reached 7.7 and 4.0 months, respectively [19]. Another retrospective analysis evaluated the safety and efficacy of venetoclax in 18 pediatric/young adult patients with R/R ALL or lymphoblastic lymphoma (LBL) showed a 61% CR rate and 9.14-month median OS. The treatment of venetoclax proved to be safe for patients while myelosuppression emerged as the primary adverse effect [20]. The EA9152 phase I trial administered venetoclax to 18 adult patients with R/R ALL/LBL who received liposomal vincristine (L-VCR). The trial established 600 mg venetoclax as the maximum tolerated dose while achieving CR in 22% of patients including MRD-negative cases with acceptable safety results [21]. Separately, a phase I trial of venetoclax plus low-dose navitoclax and chemotherapy in 47 pediatric/adult R/R ALL/LBL patients showed a 60% CR rate. Responses were observed regardless of prior allo-HCT or immunotherapy, with delayed hematopoietic recovery as the main toxicity [22]. A summary of those studies is shown in Table 1 [17-28].
 Click to view | Table 1. Published Experiences of Venetoclax in ALL |
Venetoclax plus low-intensity chemotherapy
The clinical application of venetoclax in ALL has been studied further in R/R settings by combining it with low-intensity chemotherapy. Phase 1/2 trial studied the combination of mini-hyper-CVD chemotherapy with venetoclax in adults with R/R ALL. The regimen demonstrated promising activity, achieving a composite CR and CR with incomplete hematologic recovery (CRi) rate of 57%, with 45% of responders attaining MRD negativity. The trial enrolled heavily pretreated patients, with over half having undergone prior allo-HCT, and many with prior exposure to inotuzumab ozogamicin, blinatumomab, or chimeric antigen receptor (CAR) T-cell therapy. The treatment was generally well tolerated, with infections being the most common grade ≥ 3 adverse event. Median OS was 7.1 months, and the 1-year OS was 29% [23]. These findings support the incorporation of venetoclax into low-intensity regimens as a viable therapeutic strategy in R/R ALL and warrant further validation in randomized trials.
Venetoclax with HMAs
Research findings show that venetoclax works well when used with HMAs including azacitidine or decitabine to treat R/R T-ALL patients who have few available treatment choices. Farhadfar et al published a 2021 study about a patient with relapsed early T-cell ALL with high-risk features including TP53 loss and poor chemotherapy response. The patient received venetoclax and decitabine following an allo-HCT. The patient achieved MRD-negative CR after one cycle of treatment but died from infectious complications [29]. The 2023 Chinese multicenter phase 2 trial used venetoclax in combination with azacitidine to treat 18 patients who had R/R T-ALL or T-LBL. The research study achieved an 88.9% overall response rate and MRD-negative CR in a subset of patients while patients survived for 24.1 months on average. The treatment allowed two-thirds of patients to undergo allo-HCT while hematologic toxicities occurred frequently but the regimen remained tolerable without treatment-related deaths [24].
A study by Agrawal et al evaluated the effectiveness of HMAs with venetoclax as salvage treatment for adults with R/R ETP-ALL through a case series. Three out of five patients (60%) reached CR and two of them received successful allo-HCT treatment. The responders reached MRD-negative status while the treatment with azacitidine or decitabine showed similar toxicity patterns to AML therapy [25]. The study by Jin et al (2025) presented a new triplet therapy consisting of venetoclax, chidamide (a selective histone deacetylase inhibitor), and azacitidine (VCA) for a patient with relapsed B-ALL who had the high-risk MLL-AF4 fusion and extramedullary relapse after transplant. The patient achieved CR after the first cycle and received successful CD19 CAR T-cell therapy. The case demonstrates how these combination therapies including HMA-venetoclax doublets and VCA triplet regimens function as effective bridging treatments for high-risk ALL subtypes through BCL-2 inhibition and epigenetic modulation and demethylation synergies [30].
Venetoclax in pediatric and adolescent/young adult (AYA) populations
Research studies conducted recently have expanded the use of venetoclax for treating R/R ALL in pediatric and AYA populations. A phase 1 open-label multicenter study assessed venetoclax safety and effectiveness when used with chemotherapy in pediatric and AYA patients younger than 25 who had R/R ALL. The study included 31 patients who received either venetoclax alone (n = 1), venetoclax with dexamethasone vincristine and/or pegasparaginase (VXL; n = 20) or venetoclax with cytarabine etoposide and/or pegasparaginase (n = 10). The treatment showed good tolerance from patients with febrile neutropenia being the most common grade 3/4 treatment-emergent adverse event (55%). A fatal adverse event possibly linked to venetoclax occurred in one patient. The overall response rate was 42%, with all responders achieving CR or CRi. The responses were observed in biomarker-evaluable patients regardless of the genetic alterations and BCL-2 family dependencies [26]. These results indicate that venetoclax in combination with VXL-based or cytarabine-based regimens represents a tolerable and promising salvage option for pediatric and AYA patients with R/R ALL. A recent longitudinal update from a phase 2 trial by Ravandi et al evaluated the integration of venetoclax into a frontline T-ALL/T-LBL regimen combining hyper-CVAD, nelarabine, and pegylated asparaginase. Among 145 adult patients with newly diagnosed or minimally treated T-ALL/LBL, 46 received venetoclax during induction and consolidation phases. At a median follow-up of 24.4 months, patients in the venetoclax cohort achieved superior 2-year progression-free survival (PFS) of 87.9% versus 64.1% (P = 0.03) and duration of response (DOR) of 93.6% versus 69.2% (P = 0.005), compared to the original cohort. The 2-year OS was also higher (87.8% vs. 73.9%), although this did not reach statistical significance (P = 0.16). Venetoclax addition was well tolerated, with febrile neutropenia being the most common serious adverse event (60%) [27]. These findings support the potential of venetoclax to enhance the durability of response and long-term outcomes when incorporated into frontline multi-agent regimens for T-ALL/LBL.
Venetoclax in Ph-like B-ALL subtypes
The survival of leukemic cells depends heavily on BCL-2 overexpression especially in the high-risk Ph-like ALL subtype. In a prospective study, 24 Ph-negative B-ALL patients received venetoclax at 400 mg daily for 14 days during induction and consolidation therapy with the CALGB 10403 pediatric-inspired chemotherapy regimen. The research included patients aged 31 years on average (range, 18 - 53) with Hispanic patients making up 92% of the total population. Twelve patients (50%) had Ph-like ALL. The regimen showed favorable safety profile with no dose-limiting toxicities observed during the phase 1 portion. The treatment resulted in CR or CRi in 96% (23/24) of patients after induction therapy and MRD negativity (< 0.01%) was achieved by 91% (20/22) of patients who received consolidation therapy. The Ph-like ALL patients reached MRD-negative CR/CRi status in 11 out of 12 cases while one patient maintained low-level MRD at 0.01%. The Ph-like ALL cases demonstrated higher BCL-2 dependency at baseline compared to non-Ph-like ALL patients, although the difference did not reach statistical significance (P = 0.06) [28]. The research indicates that venetoclax improves Ph-like ALL remission depth when used with pediatric-inspired regimens, thus supporting its potential use as a frontline treatment for this high-risk population.
| Mechanisms of Resistance and Strategies to Overcome Them | ▴Top |
The primary cause of venetoclax resistance in ALL stems from anti-apoptotic protein heterogeneity especially within the BCL-2 family. While venetoclax sensitivity in B-ALL is largely driven by BCL-2 overexpression, resistance commonly emerges through adaptive upregulation of alternative anti-apoptotic proteins such as MCL-1 or BCL-XL, which sequester pro-apoptotic mediators like BIM and BAX. In contrast, T-ALL, particularly mature subtypes, exhibits constitutively high BCL-XL expression and shows limited dependence on BCL-2, rendering single-agent venetoclax less effective. Only ETP-ALL demonstrates meaningful BCL-2 dependence. These lineage-specific apoptotic profiles underscore the need for rational, subtype-specific combinations, such as dual BCL-2/BCL-XL inhibition, to overcome resistance and optimize therapeutic outcomes [7, 31, 32]. The main mechanism behind this resistance involves the elevation of alternative anti-apoptotic proteins like MCL-1 and BCL-XL which form complexes with BIM and BAX, thus avoiding BCL-2 inhibition [31]. ALL subtypes including T-ALL show dependency on both BCL-2 and BCL-XL or may shift toward BCL-XL or MCL-1 dependence when their disease progresses. The dynamic modulation of survival proteins allows leukemia cells to escape apoptosis, so they can survive beyond the effectiveness of venetoclax monotherapy. The mature T-ALL group demonstrates higher BCL-XL expression which makes them less responsive to venetoclax treatment. BCL-XL expression elevates in Ph-like B-ALL through JAK-STAT signaling which makes it harder to treat these cases, because it enhances resistance to BCL-2 inhibitors. The shift in apoptotic dependencies has been validated by BH3 profiling and gene activity analyses which shows that dual inhibition of BCL-2 and BCL-XL is essential to overcome resistance and achieve effective apoptosis in refractory ALL subtypes [32].
The development of various logical combination approaches was proposed for overcoming these resistance mechanisms. The therapeutic potential of combining venetoclax with AZD0466 which is a novel dendrimer-conjugated dual BCL2/BCL-XL inhibitor derived from AZD4320 was assessed in preclinical research. The research found that B-ALL samples displayed elevated BCL2 protein levels which resulted in higher sensitivity to venetoclax and AZD4320 treatment. The T-ALL samples showed increased BCL-XL protein expression which led to better responses when treated with dual BCL2/BCL-XL blockade. The results from BH3 profiling demonstrated that BCL2 was the primary apoptotic pathway in B-ALL but T-ALL required simultaneous activation of BCL2 and BCL-XL for apoptosis. The in vivo studies demonstrated that AZD0466 reduced tumor growth and enhanced survival rates in T-ALL mouse models but venetoclax failed to deliver comparable therapeutic effects [32]. The research demonstrates that dual BCL2/BCL-XL inhibition provides better outcomes in T-ALL which supports further development of AZD0466 as a therapeutic candidate for ALL treatment.
The dual inhibitor navitoclax demonstrates therapeutic potential by extending anti-apoptotic blockade through simultaneous targeting of BCL-2 and BCL-XL to overcome resistance [8]. Clinical studies demonstrate that venetoclax becomes more effective when combined with chemotherapy and p53-activating agents like MDM2 inhibitors which leads to better apoptotic priming and treatment duration [13]. The effectiveness of venetoclax in ALL treatment depends on both dynamic apoptotic profiling and individualized combination therapy approaches to overcome drug resistance.
| Safety and Tolerability | ▴Top |
Venetoclax-based ALL treatments show an acceptable safety profile but specific adverse events occur frequently during combination therapy. The most frequent adverse events reported in clinical trials were hematologic toxicities including neutropenia, thrombocytopenia, and anemia with febrile neutropenia being the most serious complication [12, 26]. The risk of infections including bacterial, viral, and fungal types increases when patients experience prolonged neutropenia, so healthcare providers must provide strict supportive care. The reported non-hematologic toxicities included nausea and diarrhea together with transaminitis but these side effects were generally mild to moderate in severity [26].
The management of these toxicities requires both preventive supportive care and continuous clinical observation. The assessment of baseline risk together with regular complete blood counts becomes vital during the first cycles of therapy. The use of prophylactic antimicrobials should be considered for patients at high risk and granulocyte colony-stimulating factor (G-CSF) support can be given to patients who need help managing neutropenia. The initiation phase of venetoclax treatment requires careful dose escalation to minimize tumor lysis syndrome (TLS) risk particularly in patients with high disease burden; TLS prophylaxis should include aggressive hydration and uric acid-lowering agents and close metabolic monitoring [33]. Treatment interruptions or dose reductions should be implemented when patients experience prolonged cytopenias or grade ≥ 3 toxicities. The administration of venetoclax becomes safe for pediatric and adult ALL patients through appropriate precautions and supportive care measures.
| Future Directions and Ongoing Trials | ▴Top |
The clinical application of venetoclax in ALL continues to evolve, with multiple ongoing trials investigating its integration into frontline and salvage regimens across diverse ALL subtypes. Several phase 1/2 studies are currently evaluating venetoclax in combination with novel agents such as MDM2 inhibitors, JAK inhibitors, and monoclonal antibodies (e.g., blinatumomab or inotuzumab ozogamicin), particularly in high-risk phenotypes like Ph-like B-ALL, KMT2A-rearranged ALL, and ETP-ALL (Tables 2 and 3). These studies aim to identify synergistic partners that enhance venetoclax’s efficacy while preserving tolerability, especially in R/R settings or for patients ineligible for intensive chemotherapy.
 Click to view | Table 2. Ongoing Phase I Venetoclax-Based Trials in ALL |
 Click to view | Table 3. Ongoing Phase II Venetoclax-Based Clinical Trials |
In parallel, there is growing recognition of the need for robust biomarkers to predict response to venetoclax and guide rational patient selection. Dynamic BH3 profiling has emerged as a functional assay capable of assessing BCL-2 dependence and forecasting treatment sensitivity [34]. Additionally, baseline expression levels of BCL-2 family proteins [35], mutational landscapes (e.g., TP53, NRAS, NOTCH1) [36, 37], and MRD kinetics [38] are under investigation as potential predictive and prognostic indicators. Integration of such biomarkers into trial design may enable personalized therapy, optimize combination strategies, and identify mechanisms of primary or acquired resistance. As the field advances, the success of venetoclax in ALL will likely depend on a precision medicine framework that aligns targeted therapy with molecular and functional disease profiling.
| Conclusions | ▴Top |
The therapeutic agent venetoclax shows promise for treating ALL by combining with other cytotoxic, targeted or epigenetic therapies. The preclinical and early clinical data show that certain ALL subtypes including ETP-ALL, Ph-like B-ALL, and KMT2A-rearranged B-ALL require BCL-2 for survival, thus making them susceptible to venetoclax-based treatments. The combination of venetoclax with low-intensity chemotherapy and HMAs and pediatric-inspired regimens has shown promising results in clinical trials with high MRD negativity rates and acceptable safety profiles.
The clinical data support the use of venetoclax in current treatment approaches especially for R/R patients and those who cannot receive intensive chemotherapy. The drug shows potential for frontline use in high-risk genetic subgroups but requires ongoing prospective validation to confirm its effectiveness.
However, several questions remain. The mechanisms of resistance need better characterization especially the upregulation of MCL-1 and BCL-XL to develop rational combination and sequencing strategies. The development of reliable biomarkers is essential to select patients and deliver personalized treatment.
Research should concentrate on improving combination regimens and studying venetoclax with immunotherapy and implementing functional assays like BH3 profiling into standard clinical practice. The implementation of these efforts will help venetoclax establish new therapeutic benchmarks for pediatric and adult ALL patients.
Acknowledgments
The authors would like to thank the Department of Hematology & Cellular Therapy at King Faisal Specialist Hospital & Research Centre (KFSHRC) for their valuable input and support during the preparation of this manuscript. We also acknowledge the contributions of clinical and research colleagues whose insights helped refine the scientific content.
Financial Disclosure
No external funding was received for this work.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Amr Hanbali developed the concept and drafted the manuscript, while Ahmed Kotb conducted literature research and wrote sections of the paper under the supervision of Mostafa Saleh who reviewed the content critically. All authors approved the final version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577.
doi pubmed - Mullighan CG. Acute lymphoblastic leukemia. Acute Lymphoblastic Leukemia in Children and Adolescents. 2024:21.
- Iacobucci I, Mullighan CG. Genetic basis of acute lymphoblastic leukemia. J Clin Oncol. 2017;35(9):975-983.
doi pubmed - Neumann M, Heesch S, Gokbuget N, Schwartz S, Schlee C, Benlasfer O, Farhadi-Sartangi N, et al. Clinical and molecular characterization of early T-cell precursor leukemia: a high-risk subgroup in adult T-ALL with a high frequency of FLT3 mutations. Blood Cancer J. 2012;2(1):e55.
doi pubmed - Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, McCastlain K, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005-1015.
doi pubmed - Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14(6):e205-217.
doi pubmed - Seyfried F, Stirnweiss FU, Niedermayer A, Enzenmuller S, Horl RL, Munch V, Kohrer S, et al. Synergistic activity of combined inhibition of anti-apoptotic molecules in B-cell precursor ALL. Leukemia. 2022;36(4):901-912.
doi pubmed - Bell HL, Blair HJ, Jepson Gosling SJ, Galler M, Astley D, Moorman AV, Heidenreich O, et al. Combination p53 activation and BCL-x(L)/BCL-2 inhibition as a therapeutic strategy in high-risk and relapsed acute lymphoblastic leukemia. Leukemia. 2024;38(6):1223-1235.
doi pubmed - Coustan-Smith E, Kitanaka A, Pui CH, McNinch L, Evans WE, Raimondi SC, Behm FG, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996;87(3):1140-1146.
pubmed - Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A. The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias. Adv Hematol. 2012;2012:524308.
doi pubmed - Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202-208.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, Degryse S, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124(25):3738-3747.
doi pubmed - Numan Y, Alfayez M, Maiti A, Alvarado Y, Jabbour EJ, Ferrajoli A, Konoplev SN, et al. First report of clinical response to venetoclax in early T-cell precursor acute lymphoblastic leukemia. JCO Precis Oncol. 2018;2.
doi pubmed - Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, Cortes J, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4(3):362-375.
doi pubmed - Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, Hsi ED, et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6(1):e1593.
doi pubmed - Jain N, Stevenson KE, Winer ES, Garcia JS, Stone RM, Jabbour E, et al. A multicenter phase I study combining venetoclax with mini-hyper-CVD in older adults with untreated and relapsed/refractory acute lymphoblastic leukemia. Blood. 2019;134:3867.
- Venugopal S, Kantarjian H, Short NJ, Thompson PA, Pemmaraju N, Jain N, et al. A phase II study of mini-hyper-CVD plus venetoclax in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia. Blood. 2021;138:1239.
- Richard-Carpentier G, Jabbour E, Short NJ, Rausch CR, Savoy JM, Bose P, Yilmaz M, et al. Clinical experience with venetoclax combined with chemotherapy for relapsed or refractory T-cell acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(4):212-218.
doi pubmed - Gibson A, Trabal A, McCall D, Khazal S, Toepfer L, Bell DH, Roth M, et al. Venetoclax for children and adolescents with acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancers (Basel). 2021;14(1).
doi pubmed - Palmisiano N, Lee J-W, Claxton DF, Paietta E, Alkhateeb HB, Park JH, et al. Maximal tolerated dose determined for venetoclax in combination with liposomal vincristine in patients with relapsed or refractory Ph-negative T-cell or B-cell acute lymphoblastic leukemia: results of phase 1 portion of ECOG-ACRIN EA9152. Blood. 2021;138:3407.
- Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, Leonard J, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11(6):1440-1453.
doi pubmed - Short NJ, Jabbour E, Jain N, Senapati J, Nasr L, Haddad FG, Li Z, et al. A phase 1/2 study of mini-hyper-CVD plus venetoclax in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Adv. 2024;8(4):909-915.
doi pubmed - Cao HY, Chen LL, Wan CL, Hu XH, Wu B, Yang L, et al. Venetoclax combined with azacitidine was effective and safe for relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma: preliminary results of a phase 2, multicenter trial. Blood. 2023;142:1501.
- Agrawal V, Arslan S, Pourhassan H, Koller P, Aldoss I, Pullarkat V. Hypomethylating agent and venetoclax are effective salvage therapies in relapsed/refractory early T-cell precursor acute lymphoblastic leukaemia. Br J Haematol. 2025;206(6):1678-1682.
doi pubmed - Place AE, Karol SE, Forlenza CJ, Cooper TM, Fraser C, Cario G, O'Brien MM, et al. Venetoclax combined with chemotherapy in pediatric and adolescent/young adult patients with relapsed/refractory acute lymphoblastic leukemia. Pediatr Blood Cancer. 2025;72(6):e31630.
doi pubmed - Ravandi F, Senapati J, Jain N, Short NJ, Kadia T, Borthakur G, Konopleva M, et al. Longitudinal follow up of a phase 2 trial of venetoclax added to hyper-CVAD, nelarabine and pegylated asparaginase in patients with T-cell acute lymphoblastic leukemia and lymphoma. Leukemia. 2024;38(12):2717-2721.
doi pubmed - Aldoss I, Zhang J, Shimamoto K, Saygin C, Robbins M, Agrawal V, Aribi A, et al. Venetoclax in combination with a pediatric-inspired regimen for the treatment of newly diagnosed adults with Philadelphia chromosome-negative acute lymphoblastic leukemia. Haematologica. 2025;110(5):1105-1114.
doi pubmed - Farhadfar N, Li Y, May WS, Adams CB. Venetoclax and decitabine for treatment of relapsed T-cell acute lymphoblastic leukemia: A case report and review of literature. Hematol Oncol Stem Cell Ther. 2021;14(3):246-251.
doi pubmed - Jin X, Liu Z, Wu Y, Ji J. Venetoclax in combination with chidamide and azacitidine for the treatment of relapsed/refractory B-cell acute lymphoblastic leukemia with the MLL-AF4 gene: a case report and literature review. Front Immunol. 2024;15:1475974.
doi pubmed - Xu Y, Ye H. Progress in understanding the mechanisms of resistance to BCL-2 inhibitors. Exp Hematol Oncol. 2022;11(1):31.
doi pubmed - Kannan S, Li Y, Baran N, Yang X, Ghotbaldini S, Zhang Tatarata Q, Yoshimura S, et al. Antileukemia efficacy of the dual BCL2/BCL-XL inhibitor AZD0466 in acute lymphoblastic leukemia preclinical models. Blood Adv. 2025;9(3):473-487.
doi pubmed - Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322.
doi pubmed - Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106-1117.
doi pubmed - Kapoor I, Bodo J, Hill BT, Hsi ED, Almasan A. Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis. 2020;11(11):941.
doi pubmed - Johnson LD, Tang S, Zhang Y, Metzner M, Aviles L, Kavelaars F, et al. Mutational landscape and depth of response in patients with acute myeloid leukemia (AML) treated with magrolimab in combination with venetoclax and azacitidine compared to placebo. Blood. 2024;144:2914.
- Han H, Yao Y, Wang H, Zhou M, Zhang Z, Xu X, Qi J, et al. Landscape and clinical impact of NOTCH mutations in newly diagnosed acute myeloid leukemia. Cancer. 2023;129(2):245-254.
doi pubmed - Heiblig M, Requena GA, Tauveron-Jalenques U, Tavernier E, Cornillon J, Carre M, et al. Measurable residual disease (MRD) determinants, kinetics and its impact on survival in patients treated with azacitidine and venetoclax for acute myeloid leukemia in frontline setting: a multicentric study from French auraml group. Blood. 2024;144:846.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.