| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 14, Number 5, October 2025, pages 267-272
Acute Undifferentiated Leukemia Presented With Mediastinal Sarcoma
Hala Alsoukhnia, e, Dima AL-Dabbasa, Mohammad Masalhaa, Omar Shkaibib, Walla’a Aljbourc, Mohamad Obedatd
aLaboratory Department, Military Cancer Center, Amman, Jordan
bLaboratory Department, Queen Alia Military Hospital, Amman, Jordan
cRadiology Department, Military Cancer Center, Amman, Jordan
dHematology Department, Military Cancer Center, Amman, Jordan
eCorresponding Author: Hala Alsoukhni, Laboratory Department, Military Cancer Center, Amman, Jordan
Manuscript submitted June 16, 2025, accepted August 7, 2025, published online October 10, 2025
Short title: AUL Presented With Mediastinal Sarcoma
doi: https://doi.org/10.14740/jh2090
| Abstract | ▴Top |
Acute undifferentiated leukemia (AUL) is a rare, aggressive type of acute leukemia associated with poor prognosis, in which the blasts lack lineage commitment to either the myeloid or lymphoid lineages. It is classified according to the World Health Organization (WHO) classification under the category of acute leukemias of ambiguous lineage (ALAL). Here we present a case of a 20-year-old man, who was referred to Military Cancer Center in February 2025, as a case of mediastinal myeloid sarcoma, which was diagnosed by biopsy of the mass. Bone marrow examination was performed in our center and showed infiltration by blasts, flow cytometry of bone marrow aspirate specimen revealed that blasts do not express lineage-specific markers with expression of primitive markers, so the diagnosis of AUL was established. For precise diagnosis of AUL, a comprehensive and broad panel of immunomarkers should be run to rule out any lineage commitment for the clone. Review of literature revealed only one previously published similar case.
Keywords: Acute undifferentiated leukemia; Sarcoma; Flow cytometry
| Introduction | ▴Top |
Acute undifferentiated leukemia (AUL) is a rare type of acute leukemia that is characterized by the lack of expression of lineage-specific markers that distinguish myeloid, B- or T-lymphoid lineages with expression of primitive stem cell markers such as CD34, CD38, and human leukocyte antigen-DR isotype (HLA-DR), and usually positive for CD45 and CD7 [1, 2]. The lineage-specific marker for myeloid lineage is myeloperoxidase (MPO); for the B-lymphoid lineage, they are CD19, CD22 and CD79a; and for T-lymphoid lineage, it is cytoplasmic CD3 [3]. Genetic data on AUL are limited because of rarity of cases [4]. No characteristic cytogenetic abnormalities could be assigned to AUL, but the limited available data suggest that expression of genes associated with poor prognosis are often expressed, such as BAALC, ERG, and MN1; and to date, no reports have documented cases showing genetic mutations usually seen in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML), including FLT3, WT, MLL, or BCR-ABL [1]. Some studies showed cases with deletion 5, trisomy 8, trisomy 13 and trisomy 12 [5, 6]. These cases do not have well-established treatment protocol and harbor high risk of relapses with a median overall survival (OS) time of only 9 months, far lower than ALL and AML [7].
Myeloid sarcoma (MS) is extramedullary mass of myeloblasts that can occur in isolation (de novo) or with AML and myeloid neoplasms [8]. It can occur at any age and any site, but the most involved sites are skin, lymph nodes, soft tissue, and bone [9]. Fine needle aspiration (FNA) is usually inadequate for diagnosis of MS, and tissue biopsy is the preferred method [10]. Genetic aberrations in MS include monosomy 7, trisomy 8, MLL, NPM1, FTL3, inv(16), monosomy 16, loss of 16q, 5q, or 20q [1]. The treatment depends on the disease presentation, whether it is an initial diagnosis or relapse, as well as the age of the patient and performance status. Treatment options include chemotherapy, radiation, surgery and allogenic stem cell transplantation [11]. The prognosis in cases of MS at diagnosis of AML is poor, while isolated MS shows better outcomes [9, 12].
| Case Report | ▴Top |
A 20-year-old man with a history of congenital hypothyroidism and cryptorchidism presented to Al Mafraq Governmental Hospital with shortness of breath (SOB). A chest X-ray revealed right sided pleural effusion, and the patient was referred to King Abdulla University Hospital in Irbid in January 2025 for further investigations, where they placed pigtail catheter and drained exudative fluid. Echocardiogram was performed and revealed pericardial effusion, then serial echo revealed progression of pericardial effusion. Computed tomography (CT) scan was requested and showed multiple posterior mediastinal lymph nodes with pleural and pericardial effusion. Pericardiocentesis and pleurocentesis were performed for symptoms relief, and the collected fluids were sent to the laboratory for investigations. Cytology showed 90% mononuclear blastoid cells, and flow cytometry revealed positive cells for CD34, CD33, and human leukocyte antigen-DR isotype (HLA-DR), and negative for CD2, CD3, CD10, CD19, CD20, CD13, CD14, CD64, MPO and terminal deoxynucleotidyl transferase (TdT).
MS was suspected according to immunophenotype of the cells, so tru-cut biopsy for mediastinal lymph nodes was done and showed infiltration by atypical mononuclear cells with high nuclear/cytoplasmic (N/C) ratio. Immunohistochemical stains revealed tumor cells positive for CD34 and negative for all lineage-specific markers. So, the diagnosis of MS was confirmed. Positron emission tomography (PET) scan was done to determine the extent of the disease and showed increased bone marrow activity in addition to the bulky mediastinal mass.
The patient was referred to Military Cancer Center in February. Complete blood count showed leukocytosis (white blood cell (WBC) count = 15 × 106/mL) with normal hemoglobin (Hb) and platelets count, liver and kidney functions were normal, international normalized ratio (INR) was 1.53, and lactate dehydrogenase (LDH) level was 229 U/L. Bone marrow examination was performed and showed infiltration by blasts constituting around 60% of bone marrow cells (Fig. 1). A wide comprehensive flow cytometry panel was run and showed positivity for CD34, CD38, HLA-DR, CD33, and CD7 and negative for all other myeloid and lymphoid markers including MPO, CD117, CD13, CD11b, CD14, CD16, CD35, CD36, CD64, CD300e (IREM-2), CD5, CD1a, and CD10 (Fig. 2). So, the diagnosis of AUL was reported (Table 1). Cytogenetic tests showed monosomy 16 in 65% of metaphases. Cardiology consultation was requested before starting chemotherapy. Pericardiocentesis was recommended by the cardiologist, and 400 mL of serous fluid was drained. After that the patient complained about abdominal pain, so surgical consultation was sent. The abdominal CT scan with contrast was done, which revealed splenic infarction.
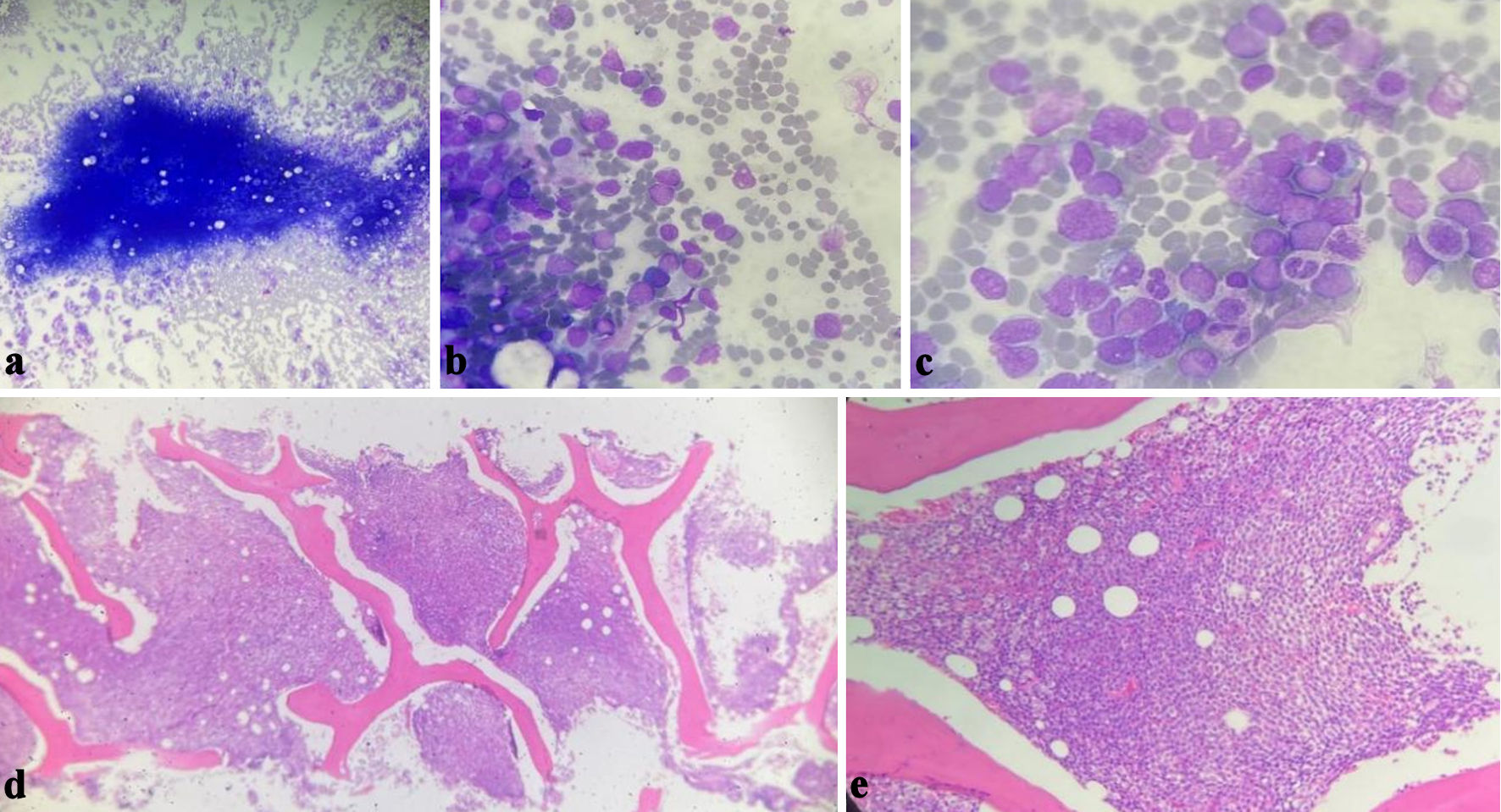 Click for large image | Figure 1. Bone marrow aspirate (a, b, c) showing the blasts infiltrating the bone marrow, the morphology is more toward myeloid leukemia. Bone marrow biopsy (d, e) showing diffuse total infiltration by blasts. |
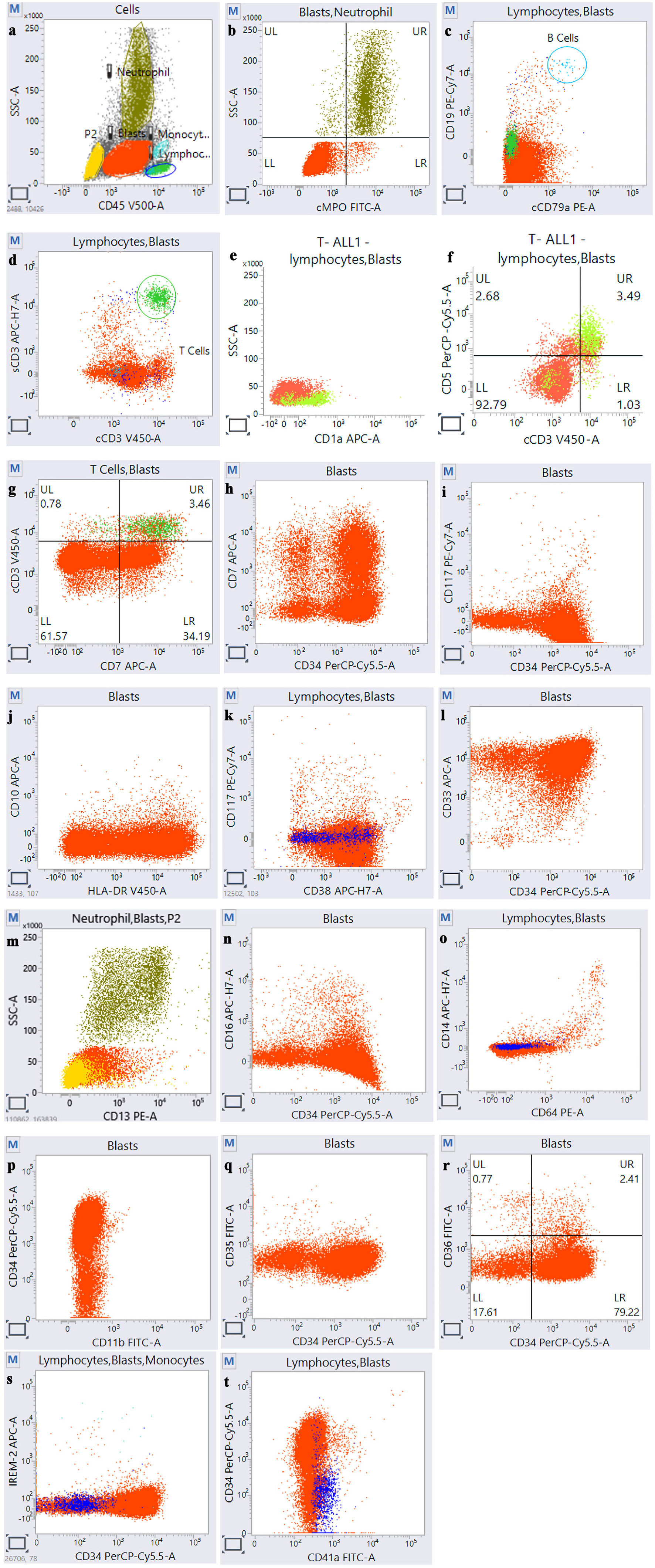 Click for large image | Figure 2. Flow cytometry of acute undifferentiated leukemia case showing blast population (red) positive for CD45 (a), negative for lineage-specific antigen (b, c, d), negative for T-cell markers (e, f), positive for CD7 (g, h), positive for primitive markers, which are CD34, HLA-DR, and CD38, and negative for CD117 (i, j, k), positive for only one myeloid marker CD33 (l), and negative for all other markers (m, n, o, p, q, r, s, t). HLA-DR: human leukocyte antigen-DR isotype. |
 Click to view | Table 1. Timeline of the Case |
Treatment was initiated with the 3 + 7 regimen, consisting of induction with cytarabine and idarubicin, followed by consolidation with high-dose cytarabine (HiDAC) regimen. Allogenic stem cell transplantation was scheduled.
Follow up-bone marrow aspirate was performed 4 weeks post induction chemotherapy and showed complete remission. The patient developed blurring of vision and headache during treatment protocol. A cerebrospinal fluid (CSF) test for blasts was performed and showed negative results, and brain CT scan and magnetic resonance imaging (MRI) were suggestive of fungal sinusitis. Ophthalmologic and ear, nose and throat (ENT) consultations were performed, and the patient was started on antifungal treatment and high-dose steroid.
The patient continued consolidation until the donor for allogenic transplant was found; but unfortunately, the patient went to Al-Mafraq Governmental Hospital in May complaining of SOB and vomiting. His WBC count was 0.3 × 106/mL, Hb was 6.8 g/dL and platelets count 2 × 106/mL. He was admitted to the hospital, and few hours later he developed loss of consciousness. Urgent brain CT scan was performed and revealed brain hemorrhage with edema and brain stem herniation; the patient died on the same day.
| Discussion | ▴Top |
In the 2016 update of the World Health Organization (WHO) classification of myeloid neoplasms, AUL is considered a subcategory of acute leukemias of ambiguous lineage (ALAL). This rare subtype of acute leukemia lacks evidence of either lymphoid or myeloid lineage or displays evidence of more than one lineage [1]. AUL lacks the main lineage-specific immunophenotypic markers, including MPO, cCD3, CD19, CD22 and CD79a, and mostly expresses only one lineage marker, such as CD13, CD33, and CD7, in addition to stem cell markers, which represent the immature characteristic of the clone, including CD34, CD38, HLA-DR and TdT [13].
AUL is extremely rare with an incidence rate of approximately 1.34 persons/million, and it is more frequent in older age. With the advancement of diagnostic technology, the incidence has significantly decreased [14]. Compared to other subtypes of acute leukemias, AUL is often associated with worse prognosis [15].
Diagnosis of AUL mainly depends on bone marrow and flow cytometry to investigate the leukemic cells and classify the type of leukemia. Before classifying acute leukemia as undifferentiated, it is essential to perform a comprehensive immunophenotyping panel to exclude any lineage commitment [1]. Weinberg et al compared AUL with minimally differentiated AML-M0, which can be easily confused with AUL, and reported no difference in terms of age, blood count, bone marrow findings, OS, relapse-survival, and rates of complete remission [16]. In terms of immunophenotyping, AML-M0 lacks the specific myeloid marker (MPO) and expresses at least two myeloid-associated markers, while AUL expresses single, relatively nonspecific myeloid-associated marker, along with the primitive stem cell markers. ALAL, not otherwise specified, can also be confused with AUL, in which expression of more than one membrane antigen of certain lineage is expressed [1, 17]. Similar to our case, Luo et al reported a case of a patient diagnosed with AUL and MS. In their case, the blasts expressed low CD45, CD34, CD33, and CD7 but not CD2, CD10, CD5, CD4, CD8 and CD117 [17]. In our case the cells expressed the same markers but with higher CD45 expression. Cytogenetic abnormalities in AUL are not well recognized because of rarity of cases, but the reported cases showed -5q, +8, +13, Ph+, 11q23 and i(17) [15-19]. The only cytogenetic abnormality that could be detected in our patient was monosomy 16, which can occur in MS.
MS is also known as extramedullary AML and chloroma. The incidence varies among different studies ranging from 2.5% to 30% of all AML cases. MS can present as isolated disease, as a presentation of AML, or as a disease progression in myelodysplastic syndrome (MDS), myeloproliferative neoplasm (MPN), and MDS/MPN [9]. When MS precedes the hematological malignancy, it can be confused with lymphoma, and as MS rarely occurs in mediastinum, this causes more confusion and raises the suspicion of lymphoma. Paul et al [20] and Akkaya et al [21] have reported two cases of young patients, who presented with mediastinal sarcoma and were misdiagnosed as lymphomas and later found to be MS. This error can be contributed to rarity of these cases, so careful morphological examination and well-directed immunohistochemical stains for myeloid lineage markers will exclude the diagnosis of lymphoma [20, 21].
The mechanism of MS development is not clear, but the presence of homing signal that allows the blast cells to re-localize to secondary sites is considered the main explanation of this migration. MS is usually positive for myeloid and monocytic antigens such as CD33, CD68 and immature markers such as CD34 and CD117. CD99, TdT and CD56 can be detected. Lineage-specific markers for T cells and B cells should be negative to exclude the possibility of lymphoma [22].
MS is common in AML-M0, AML-M4 and AML-M5. MS in AUL is very rarely seen as only one case was reported in literature, where immunohistochemical stains of mass biopsy and flow cytometry of bone marrow specimen confirmed the same tumor cells in both sites [17]. In our patient, the positive immunohistochemical stains of mediastinal lymph node biopsy was consistent with the findings from flow cytometry of the blasts in the bone marrow.
This case report highlights the possibility of rare subtypes of acute leukemia with rare presentation and unusual site of involvement to raise the index of suspicion and keep in mind such cases.
Conclusions
For precise diagnosis of AUL, a comprehensive and broad panel of immunomarkers should be run to rule out any lineage commitment for the clone.
Acknowledgments
None to declare.
Financial Disclosure
The authors have nothing to disclose.
Conflict of Interest
None to declare.
Informed Consent
Ethical approval was obtained from the Royal Medical Services Ethical Committee. The patient provided informed consent for publication of this case report; submission does not include images that may identify the person.
Author Contributions
Dr. Hala Alsoukhni: bone marrow aspirate interpretation, literature review, and manuscript writing. Dr. Dima AL-Dabbas: data collection. Dr. Mohammad Masalha: flow cytometry analysis. Dr. Omar Shkaibi: bone marrow biopsy interpretation. Dr. Walla’a Aljbour: radiology interpretation. Dr. Mohammad Obaidat: treatment and follow-up. All the authors have read and approved the manuscript.
Data Availability
The datasets analyzed during the current study are available from the corresponding author.
| References | ▴Top |
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC; 2017.
- Qasrawi A, Victor G, Munker R. Acute undifferentiated leukemia. Blood 2019;134(1):3847.
- Brunning RD. Classification of acute leukemias. Semin Diagn Pathol. 2003;20(3):142-153.
doi pubmed - Manola KN. Cytogenetic abnormalities in acute leukaemia of ambiguous lineage: an overview. Br J Haematol. 2013;163(1):24-39.
doi pubmed - Patel SS, Weinberg OK. Diagnostic workup of acute leukemias of ambiguous lineage. Am J Hematol. 2020;95(6):718-722.
doi pubmed - Krayem B, Zuckerman T, Frisch A, Slouzkey I, Azoulay T, et al. The ELN 2022 risk stratification has no impact on the dismal prognosis of patients with acute undifferentiated leukemia. Blood. 2023;142(1):4221.
- Cui Y, Mi R, Chen L, Wang L, Li D, Wei X. Case report: Venetoclax plus Azacitidine in treatment of acute undifferentiated leukemia. Hematology. 2024;29(1):2293494.
doi pubmed - Ramia de Cap M, Chen W. Myeloid sarcoma: an overview. Semin Diagn Pathol. 2023;40(3):129-139.
doi pubmed - Claerhout H, Van Aelst S, Melis C, Tousseyn T, Gheysens O, Vandenberghe P, Dierickx D, et al. Clinicopathological characteristics of de novo and secondary myeloid sarcoma: a monocentric retrospective study. Eur J Haematol. 2018;100(6):603-612.
doi pubmed - Solh M, Solomon S, Morris L, Holland K, Bashey A. Extramedullary acute myelogenous leukemia. Blood Rev. 2016;30(5):333-339.
doi pubmed - Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013;3(4):265-270.
pubmed - Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2(5):309-316.
doi pubmed - Hayashino K, Matsuda M, Fujishita K, Iwata J, Mizobuchi M, Uemura M, Yorita K, et al. Acute undifferentiated leukemia limited to neck lymph nodes and a large mediastinal mass. J Clin Exp Hematop. 2022;62(4):222-225.
doi pubmed - Qasrawi A, Gomes V, Chacko CA, Mansour A, Kesler M, Arora R, Wei S, et al. Acute undifferentiated leukemia: data on incidence and outcomes from a large population-based database. Leuk Res. 2020;89:106301.
doi pubmed - Cortez AC, Costa AD, Bovelenta VDA, Nascimento MM, Schmidt-Filho J, et al. Acute undifferentiated leukemia with BCR-ABL translocation: case report and literature review. Transfusion and Cell Therapy. 2021;43(1):150.
- Weinberg OK, Hasserjian RP, Baraban E, Ok CY, Geyer JT, Philip J, Kurzer JH, et al. Clinical, immunophenotypic, and genomic findings of acute undifferentiated leukemia and comparison to acute myeloid leukemia with minimal differentiation: a study from the bone marrow pathology group. Mod Pathol. 2019;32(9):1373-1385.
doi pubmed - Luo L, Wang X, Luo J, Zheng S, Gong N, He Y, Xi Q, et al. Acute undifferentiated leukemia with undifferentiated myeloid sarcoma: case report and literature review. Medicine (Baltimore). 2024;103(4):e36948.
doi pubmed - Gupta N, Pawar R, Banerjee S, Brahma S, Rath A, Shewale S, Parihar M, et al. Spectrum and immunophenotypic profile of acute leukemia: a tertiary center flow cytometry experience. Mediterr J Hematol Infect Dis. 2019;11(1):e2019017.
doi pubmed - Kakimoto A, Otsubo K, Hanawa M, Kuwabara T, Tomoko Futaki-Sanbe T, et al. Acute undifferentiated leukemia or minimally differentiated acute myeloid leukemia: further emphasis on molecular analysis in leukemia diagnosis. Juntendo Medical Journal. 2016;62(1):37-41.
- Paul J S, Gandhi J, Sawhney J, Patel T, Trivedi T. Myeloid sarcoma: a mediastinal masquerader. Journal of Medical Sciences and Health. 2023;9(1):121-124.
- Akkaya B, Ozel E, Karadogan I, Bekoz H, Karpuzoglu G. Mediastinal granulocytic sarcoma. Turkish Journal of Pathology. 2008;24(3):183-185.
- Kahali B. Myeloid sarcoma: the other side of acute leukemia. Hematology - Latest Research and Clinical Advances. Guenova M, Balatzenko G (ed): IntechOpen, London, UK; 2018.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.