| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 000, Number 000, July 2025, pages 000-000
Does Time Matter From Diagnosis to Induction in Acute Myeloid Leukemia?
Osama Batayneha, f , Deevyashali Parekhb, Margarita Vazquez Almontec, Cristina Hamacherd, Angela Guptac, Danielle Passafiumec, Michael Sandhue, Carina Hernandezb, Safa Afridib, Sanjay Rao Gergal GopalakrishnaRaob, Alyssa Cortesea, Alexandra Goodmana, Kelsey Christoc, Josh Wallacec, Teresa Gentilea
aDepartment of Hematology and Oncology, SUNY Upstate Medical University, Syracuse, NY, USA
bDepartment of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY, USA
cSUNY Upstate Medical University, Syracuse, NY, USA
dDepartment of Internal Medicine, Brown University/Kent Hospital, Providence, RI, USA
eDepartment of Hematology/Oncology, Montefiore Medical Center, New York City, NY, USA
fCorresponding Author: Osama Batayneh, Department of Hematology and Oncology, SUNY Upstate Medical University, Syracuse, NY, USA
Manuscript submitted April 27, 2025, accepted June 17, 2025, published online July 8, 2025
Short title: TDT in AML
doi: https://doi.org/10.14740/jh2075
| Abstract | ▴Top |
Background: While molecular and cytogenetic testing may change prognosis and guide treatment intensity for patients with acute myeloid leukemia (AML), timing from diagnosis to treatment (TDT) on the other hand may impact treatment outcomes and survival. These considerations are sometimes at odds with each other given that molecular studies can take up to 2 weeks to result.
Methods: A retrospective cohort analysis was conducted at SUNY Upstate University Hospital to examine the effect of TDT on complete remission (CR) and overall survival (OS). The subjects were at least 18 years old and diagnosed with AML and treated between January 2010 and June 2024. TDT was divided into three categories: chemotherapy induction within 1 - 5, 6 - 10, and 11+ days. Univariate Kaplan-Myer survival analysis and multivariate Cox regression model were performed.
Results: A total of 187 patients were included, and 34% (n = 64) were younger than age 60. Patients were classified as 20% (n = 37) favorable, 36% (n = 67) intermediate, and 40% (n = 74) adverse risk, , while 4% risk stratification could not be completed due to missing data. Seventy-two percent (n = 134) had de novo AML. Chemotherapy induction began for 70% (n = 130) on days 1 - 5, 16% (n = 30) between days 6 - 10, and 14% (n = 27) on day 11 or after. The probability of achieving CR decreases for those who had induction 11+ days from diagnosis compared to those who had induction 1 to 5 days from diagnosis. This relationship was statistically significant (odds ratio = 0.32, 95% confidence interval (CI): 0.125 - 0.796; P = 0.003). However, no differences in OS and CR between TDT groups were seen when multivariate analysis was performed.
Conclusion: Our retrospective study showed no difference in OS based on TDT groups, which supports clinicians’ approach to await on comprehensive AML profiling for an optimal risk stratification at diagnosis and implementing best course of action.
Keywords: Acute myeloid leukemia; Timing from diagnosis to treatment; Complete remission; Overall survival
| Introduction | ▴Top |
Acute myeloid leukemia (AML) is an aggressive hematological malignancy, which accounts for approximately 80% of acute leukemias [1, 2]. Without treatment, the median survival ranges from a few weeks to several months [3]. Leukemia develops from the serial acquisition of somatic mutations in hematopoietic stem and progenitor cells with the capacity to regenerate the neoplastic clone [4, 5]. AML is a very heterogenous disease [6, 7]. Recent advances and our better understanding of the pathogenesis, molecular testing, and the development of novel therapies have brought us to a new era regarding the diagnosis, classification, and treatment of patients with AML [7]. Within the past few decades, genomic studies based on next-generation sequencing (NGS) have further dissected the molecular profile of AML and changed the landscape of AML treatment [4, 5]. The accumulation of molecular information provides a powerful understanding and prognostic insight about AML. An international expert panel from the European LeukemiaNet (ELN) published a well-validated risk stratification tool which is largely based on the comprehensive molecular and cytogenetic analysis at the time of diagnosis for patients receiving intensive chemotherapy [8], as well as an updated molecular risk stratification for patients receiving less intensive chemotherapy [9].
Morphological diagnosis of AML can be made in a few hours; however, results of comprehensive molecular and cytogenetic analysis on the other hand can take longer processing time, up to 2 weeks. Historically, the general recommendation is to initiate induction chemotherapy immediately after the diagnosis is confirmed due to the aggressive and acute nature of AML, especially for those with hyperleukocytosis [10]. Many clinicians wait for comprehensive molecular testing before initiating systemic treatment, utilizing hydroxyurea (HU) as a bridge for blast reduction to stabilize patients with hyperleukocytosis [11]. The optimal timing from diagnosis to treatment (TDT) remains unclear, perhaps due to the lack of prospective randomized clinical trials looking into TDT and corresponding outcomes. A comprehensive molecular and cytogenetic analysis at diagnosis can impact treatment decision and intensity [8, 9, 12-15], even for older patients [16, 17]. This is observed especially in the era of newer and targeted therapies, like liposomal daunorubicin and cytarabine (Vyxeos) for high risk/secondary AML [18], venetoclax combined with hypomethylating agents [2, 19-22], gemtuzumab ozogamicin (GO) for favorable risk AML [23], FLT3 inhibitors with or without chemotherapy for AML with FLT3 mutation [24-26], ivosidenib and azacitidine in IDH1-mutated AML [27], IDH2 inhibition in IDH-mutated AML [28], and menin inhibitors targeting driver mutations in AML such as KMT2A and NPM1 [29].
In this study, we performed a retrospective analysis to assess the complete remission (CR) and overall survival (OS) in relation to the timing of treatment initiation.
| Materials and Methods | ▴Top |
A retrospective cohort analysis was conducted at SUNY Upstate University Hospital using EPIC’s Slicer Dicer search tool, and records were reviewed in EPIC from January 2010 to June 2024. We selected all patients fulfilling the following criteria: at least 18 years old; diagnosed with AML; who received intensive or non-intensive chemotherapy. Patients with acute promyelocytic leukemia and those who did not receive any chemotherapy were excluded. The study protocol was approved by SUNY Upstate Medical University’s Institutional Review Board Committee. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Patients’ characteristics were analyzed by descriptive statistical methods. CR and early death (ED) were expressed as percentage and compared by the χ2 test, whereas for OS, univariate Kaplan-Meier survival curves were plotted to estimate survival over time for each group, with differences assessed by the log-rank test. Pearson’s Chi-square tests were performed for categorical variables. A multivariate Cox proportional hazards model was used to evaluate the independent effects of each covariate and time to death. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were reported while controlling for all other predictors. A significance level of 0.05 was used for all analyses. Likelihood ratios were calculated using logistic regression model. All statistical calculations were done using Statistical Analysis System (SAS) software, version 9.4. OS was calculated from diagnosis until death from any cause. For patients who did not die during the follow-up period, OS was censored on the date of last known follow-up. TDT was defined as time from diagnosis of AML (confirmation by peripheral blood or bone marrow samples) and the beginning of chemotherapy. Use of HU was allowed prior to initiating systemic treatment and was not counted into treatment initiation. Response criteria post chemotherapy induction were evaluated and categorized into CR and no response: CR (CR with incomplete hematological recovery (CRh) and CR with partial hematological recovery (CRi) considered as CR) and partial response was considered as no response.
TDT was stratified into three cohorts: chemotherapy induction between 1 - 5, 6 - 10, and 11+ days. Based on the ELN 2022 risk stratification system, patients were divided into three risk categories: favorable, intermediate, or adverse.
| Results | ▴Top |
Patients
The characteristics of the 187 patients and the comparison of TDT are presented in Table 1. The median age at diagnosis was 63 years old. Of the patients, 34% were younger than age of 60, 50% were males, and 82% were White. Seventy-two percent had de novo AML. According to the ELN 2022 risk stratification, 20%, 36%, and 40% of patients had favorable, intermediate, and adverse risk, respectively. ELN risk stratification could not be calculated in 4% of the patients. In our institution, the median times for NGS and karyotype results were found to be 11 - 14 and 7 - 14 days, respectively. Fifty out of 187 (27%) patients had white blood cell (WBC) counts > 50 (× 103/µL) at diagnosis. The majority of them (96%) had early treatment initiation (TDT 1 - 10), while only two patients (4%) had delayed treatment initiation (TDT 11+).
 Click to view | Table 1. Descriptive Characteristics of Patients and Comparison of TDT |
Univariate analysis of TDT and OS
The median TDT was 5.4 days. Notably, the majority of patients (n = 130) received chemotherapy within days 1 - 5. Intensive chemotherapy in our study was administered to 62% (n = 116) and included 7 + 3, Vyxeos and FLAG-Ida regimens. Non-intensive chemotherapy was administered to 37% (n = 71) and included the following: venetoclax combined with hypomethylating agents, hypomethylating agents alone, and low-dose cytarabine. Table 2 presents the OS for all TDT groups. The median OS was 20.5 months for TDT 1 - 5, 18 months for TDT 6 - 10, and 10.4 months for TDT 11+.
 Click to view | Table 2. Overall Survival in Months by Time to Induction for All Patients |
HR for OS vs. TDT is shown in Table 3. An induction time of 1 - 5 days decreased the risk of death compared to an induction time of 11 days or more, which showed statistical significance (HR = 0.567, 95% CI: 0.325 - 0.989). There was no statistical difference in OS between the days 1 - 5 and 6 - 10 (HR = 1.072, 95% CI: 0.577 - 1.992). Kaplan-Meier estimates of OS according to TDT are illustrated in Figure 1.
 Click to view | Table 3. HR for Overall Survival vs. Time to Induction |
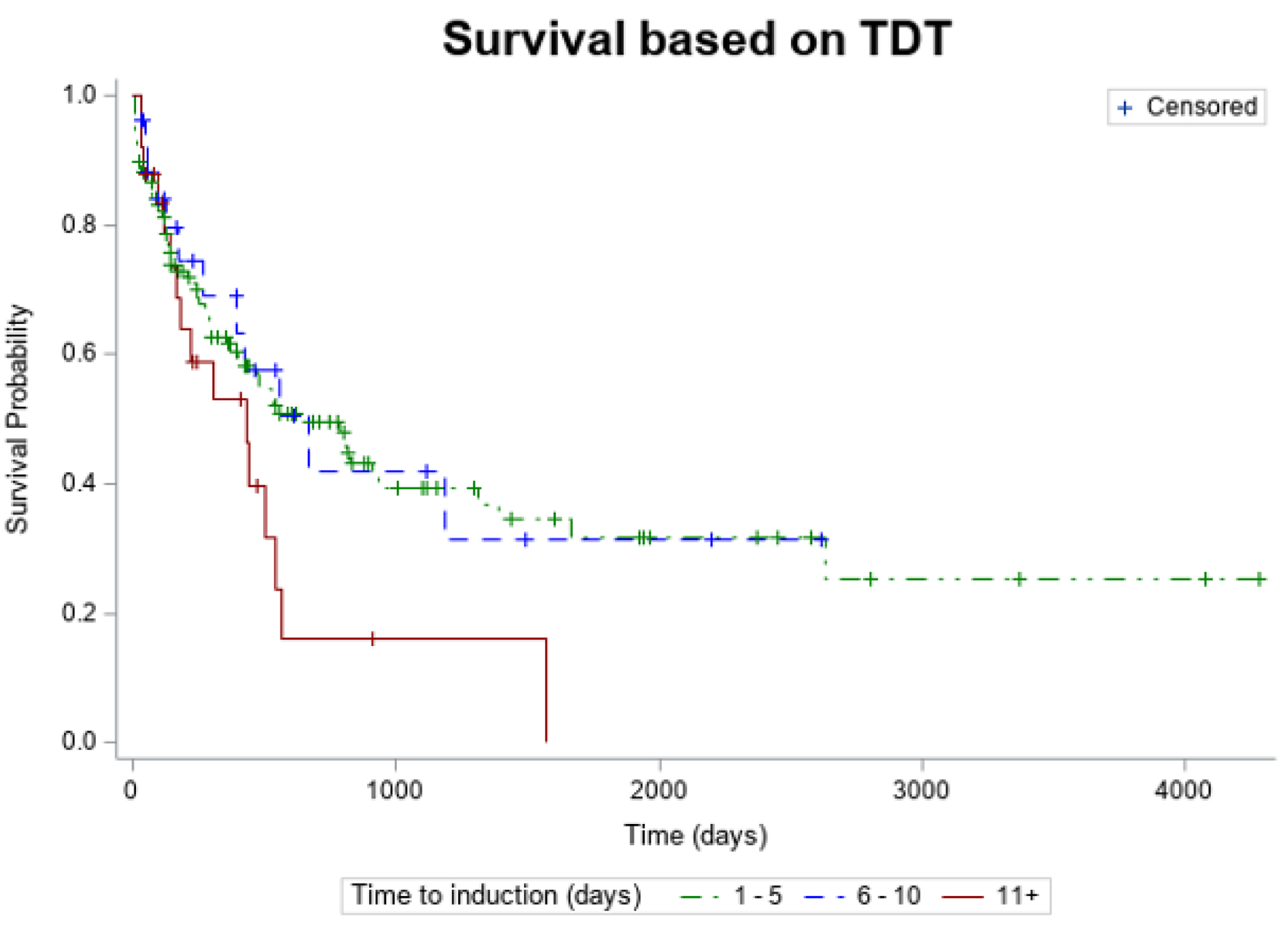 Click for large image | Figure 1. Kaplan-Meier estimates of overall survival according TDT. TDT: timing from diagnosis to treatment. |
Table 4 presents the reasons for delay in initiating chemotherapy 11+ days after diagnosis.
 Click to view | Table 4. Reasons for Delay in Initiating Chemotherapy 11+ Days After Diagnosis |
Remission status
The logistic regression model that predicted remission status using timing to induction was statistically significant (χ2(2) = 6.440, P = 0.040). Our model suggested that the odds of CR decreased in those who had induction 11+ days from diagnosis by 0.3 times compared to those who had induction 1 to 5 days from diagnosis. This relationship was statistically significant (OR = 0.32, 95% CI: 0.125 - 0.796; P = 0.003). Our model also suggested that the odds of CR increased in those who began induction 6 to 10 days from diagnosis by 1.1 times compared to those who had induction 1 to 5 days from diagnosis; however, this relationship was not statistically significant (OR = 1.13, 95% CI: 0.442 - 2.907; P = 0.157).
Multivariate analysis of TDT and OS
A multivariate analysis was performed to evaluate the independent effects of each covariate and survival. There was no statistical significance in survival between TDT 1 - 5, 6 - 10, or 11+ days. Patients younger than age of 60 trended toward better survival but without statistical significance. Notably, based on the year of diagnosis, patients with a diagnosis of AML in 2020 and after had a better OS. Our model suggested that patients who were diagnosed between 2015 and 2020 had an increased risk of death by 2.2 times compared to patients who were diagnosed in 2020 or later after controlling for all other predictors. This relationship was statistically significant (HR = 2.157, 95% CI: 1.311 - 3.575). Our model also suggested that patients who were diagnosed in 2020 or later had a decreased risk of death by 0.4 times compared to patients who were diagnosed before 2015 after controlling for all other predictors. This relationship was also statistically significant (HR = 0.395, 95% CI: 0.203 - 0.802). Figure 2 presents a forest plot for multivariate analysis, and Table 5 presents the HRs.
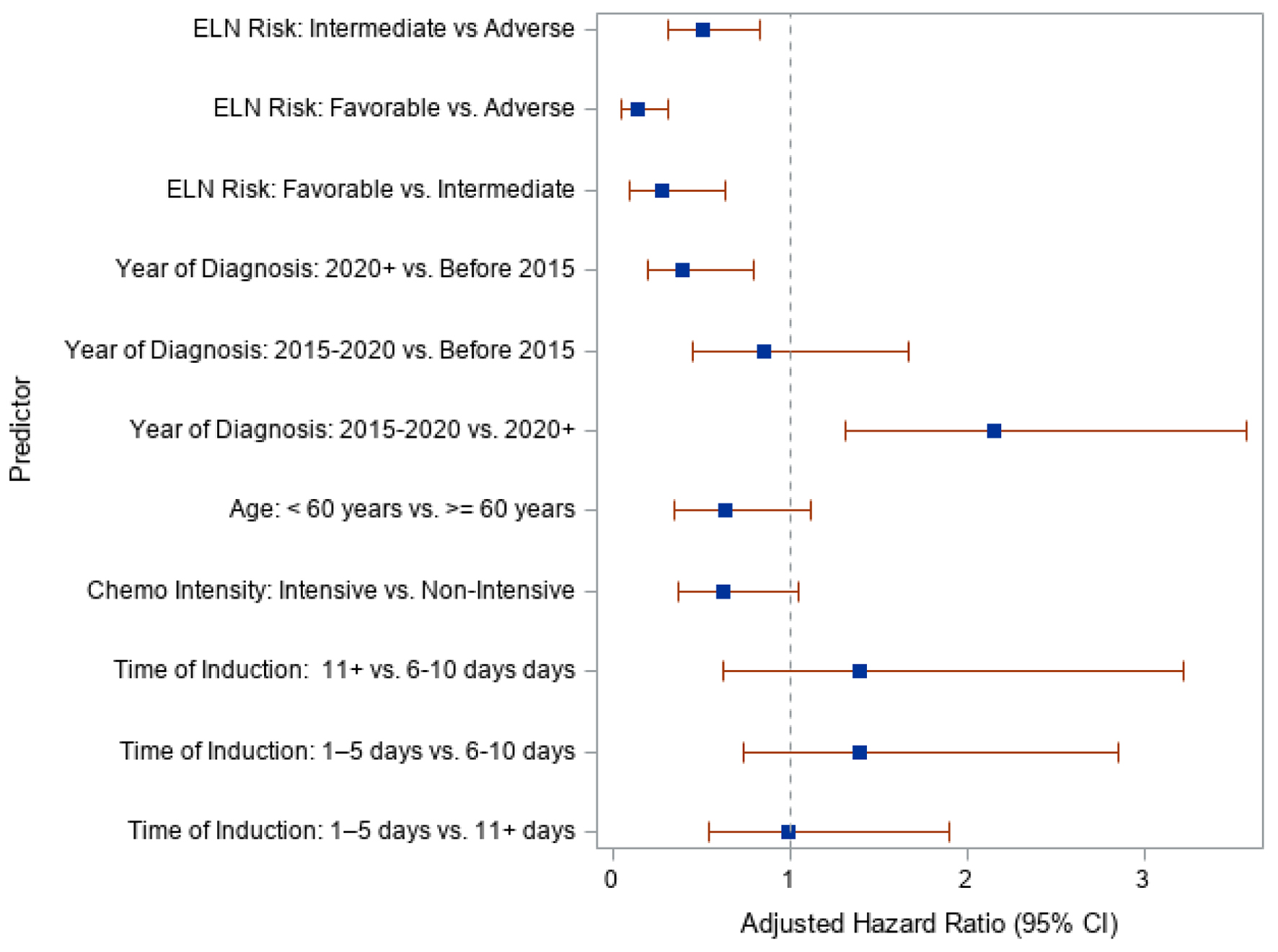 Click for large image | Figure 2. Forest plot of adjusted hazard ratios and 95% confidence interval for each predictor. |
 Click to view | Table 5. aORs for Overall Survival vs. Predictors |
TDT and ED
Amongst the 90 deaths recorded during the follow-up period, there were 13 deaths within 30 days, all of which occurred in TDT 1 - 5 group. No EDs within 30 days were seen in TDT 6 - 10 or 11+.
Table 6 presents the EDs within 30 days, within 90 days, and after 90 days.
 Click to view | Table 6. Early Death by Time to Induction for AML |
Table 7 summarizes the remission status, chemotherapy intensity, days to platelet and neutrophil recovery, and transplant status for different TDT groups.
 Click to view | Table 7. Clinical Outcomes Based on TDT Groups |
| Discussion | ▴Top |
Traditionally, the general recommendations are to start chemotherapy induction for AML patients as soon as possible due to the worries of poor outcomes when delaying therapy or untreated AML as shown in previous observational series [3, 30]. Though thus far, there have been no prospective randomized clinical trials to explicitly confirm the optimal timing to initiating treatment, perhaps due to ethical reasons.
In review of available studies that have investigated TDT and clinical outcomes, some limitations are noted. An Eastern Cooperative Oncology Group (ECOG) study in 300+ AML patients older than 55 years showed significantly lower CR with treatment delay, but no difference in OS, though patients with secondary AML were excluded in this study [30]. A mutual study between Cleveland Clinic and the MD Anderson Cancer Center that included 1,317 AML patients showed no difference in OS and remission status, except for younger patients in whom treatment was delayed by 5 days or more, both OS and CR were significantly lower, though in this study patients with leukocytosis > 50 × 109/L were excluded [31], and almost half of the patients had secondary AML which is proportionally higher than the reported percentage in the literature. Another retrospective study done in Toulouse center, France including 599 patients without excluding patients with high WBC counts showed no difference in OS or CR in both univariate and multivariate analyses between all TDT groups [32]. In a large German study that included 2,263 AML patients, the median TDT was 3 days, the majority of patients had de novo AML (75%), and no significant difference in outcomes (remission status, OS, ED) was observed between TDT groups [3]. A large systematic review of 13 studies showed highly variable results [33].
In our study, 72% had de novo AML, which is very similar to the available literature. Patients with leukocytosis > 50 × 109/L were not excluded, and use of HU prior to initiating chemotherapy was allowed. The majority of patients (48/50, 96%) with leukocytosis > 50 × 109/L were treated early (TDT 1 - 10) and only two patients (4%) had delayed treatment initiation 11+ days after diagnosis in order to achieve cytoreduction prior to initiating venetoclax-based regimen to avoid tumor lysis syndrome [34]. Although the two patients with hyperleukocytosis who had delayed treatment had OS > 6 months, it is not advised to delay initiating cytoreductive therapy for patients with hyperleukocytosis to avoid complications like spontaneous tumor lysis syndrome(TLS) and organs failure [35-37].
The majority of patients (70%) received induction chemotherapy within 5 days from diagnosis, which has shown superior CR and OS - only in univariate analysis - in comparison to patients who had treatment induction after 10 days from diagnosis; however, no difference in OS was seen between TDT groups when multivariate analysis was performed. In comparison to patients who initiated treatment early (TDT 1 - 5), patients who initiated therapy later (TDT 11+) were less likely to achieve CR (45% vs. 73%; P < 0.05), though they were older (mean 71.2 vs. 61.9), less likely to receive intensive chemotherapy (30% vs. 71%), less likely to receive allogeneic stem cell transplant (11% vs. 75%), and had higher TP53 mutations/deletions (15% vs. 5%). This suggests that age is a potential confounder. Thirteen EDs within 30 days were reported in TDT 1 - 5, whereas none were reported in TDT 6 - 10 or 11+, though no statistical correlation could be made due to small sample size. Notably, patients who were initiated on treatment in 2020 and after had better OS which likely is attributed to our better understanding of AML molecular profiling, improvement in supportive care, and the development of targeted therapies (e.g., FLT3 inhibitors, menin inhibitors, and others).
In conclusion, with the availability of newer and targeted therapies for AML, our best course of action and treatment recommendations are increasingly based on molecular profiling, highlighting the importance of obtaining and implementing comprehensive profiling at diagnosis for optimal risk stratification and management. Concordant to other studies listed above, our study suggests no statistically significant difference in OS based on timing to chemotherapy initiation.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Osama Batayneh and Teresa Gentile contributed to the study design, literacy search, conceptualization, methodology, writing and editing the manuscript, data collection and analysis. Deevyashali Parekh, Michael Sandhu, and Carina Hernandez contributed to the literacy search, conceptualization, methodology, writing and editing the manuscript, and data collection. Margarita Vazquez Almonte, Angela Gupta, and Danielle Passafiume contributed to the literacy search, conceptualization, writing and editing the manuscript, and data collection. Cristina Hamacher contributed to the literacy search, conceptualization, methodology, writing and editing the manuscript. Safa Afridi and Sanjay Rao Gergal GopalakrishnaRao contributed to the literacy search, methodology, writing and editing the manuscript, and data collection. Alyssa Cortese and Alexanda Goodman contributed to the literacy search, methodology, data collection, writing and editing the manuscript. Kelsey Christo contributed to the literacy search, methodology, conceptualization, writing and editing the manuscript. Josh Wallace contributed to the study design, conceptualization, methodology, data analysis, creating tables and graphs.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Jani CT, Ahmed A, Singh H, Mouchati C, Al Omari O, Bhatt PS, Sharma R, et al. Burden of AML, 1990-2019: estimates from the global burden of disease study. JCO Glob Oncol. 2023;9:e2300229.
doi pubmed - DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17.
doi pubmed - Rollig C, Kramer M, Schliemann C, Mikesch JH, Steffen B, Kramer A, Noppeney R, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136(7):823-830.
doi pubmed - Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059-2074.
doi pubmed - Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221.
doi pubmed - Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487-494.
doi pubmed - Guijarro F, Garrote M, Villamor N, Colomer D, Esteve J, Lopez-Guerra M. Novel tools for diagnosis and monitoring of AML. Curr Oncol. 2023;30(6):5201-5213.
doi pubmed - Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, Ebert BL, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377.
doi pubmed - Dohner H, DiNardo CD, Appelbaum FR, Craddock C, Dombret H, Ebert BL, Fenaux P, et al. Genetic risk classification for adults with AML receiving less-intensive therapies: the 2024 ELN recommendations. Blood. 2024;144(21):2169-2173.
doi pubmed - Lowenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, Cauchie C, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7(9):1268-1274.
doi pubmed - Grund FM, Armitage JO, Burns P. Hydroxyurea in the prevention of the effects of leukostasis in acute leukemia. Arch Intern Med. 1977;137(9):1246-1247.
pubmed - Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325-4336.
doi pubmed - Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, Patil SR, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58(18):4173-4179.
pubmed - Byrd JC, Ruppert AS, Mrozek K, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, et al. Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): results from CALGB 8461. J Clin Oncol. 2004;22(6):1087-1094.
doi pubmed - Byrd JC, Dodge RK, Carroll A, Baer MR, Edwards C, Stamberg J, Qumsiyeh M, et al. Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol. 1999;17(12):3767-3775.
doi pubmed - Estey E. What is the optimal induction strategy for older patients? Best Pract Res Clin Haematol. 2011;24(4):515-522.
doi pubmed - Dohner H, Dolnik A, Tang L, Seymour JF, Minden MD, Stone RM, Del Castillo TB, et al. Cytogenetics and gene mutations influence survival in older patients with acute myeloid leukemia treated with azacitidine or conventional care. Leukemia. 2018;32(12):2546-2557.
doi pubmed - Alfayez M, Kantarjian H, Kadia T, Ravandi-Kashani F, Daver N. CPX-351 (vyxeos) in AML. Leuk Lymphoma. 2020;61(2):288-297.
doi pubmed - Shimony S, Garcia JS, Keating J, Chen EC, Luskin MR, Stahl M, Neuberg DS, et al. Molecular ontogeny underlies the benefit of adding venetoclax to hypomethylating agents in newly diagnosed AML patients. Leukemia. 2024;38(7):1494-1500.
doi pubmed - Bataller A, Bazinet A, DiNardo CD, Maiti A, Borthakur G, Daver NG, Short NJ, et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 2024;8(4):927-935.
doi pubmed - DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - Wei AH, Loo S, Daver NG. How I treat patients with AML using azacitidine and venetoclax. Blood. 2025;145(12):1237-1250.
- Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, Estey EH, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986-996.
doi pubmed - Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464.
doi pubmed - Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54-60.
doi pubmed - Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103(10):3669-3676.
doi pubmed - Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, Heuser M, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519-1531.
doi pubmed - Stein EM. IDH2 inhibition in AML. Blood. 2023;141(2):124-125. Blood. 2023;141(15):1896.
doi pubmed - Huls G, Woolthuis CM, Schuringa JJ. Menin inhibitors in the treatment of acute myeloid leukemia. Blood. 2025;145(6):561-566.
doi pubmed - Rowe JM, Neuberg D, Friedenberg W, Bennett JM, Paietta E, Makary AZ, Liesveld JL, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood. 2004;103(2):479-485.
doi pubmed - Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, Kantarjian HM, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
doi pubmed - Bertoli S, Berard E, Huguet F, Huynh A, Tavitian S, Vergez F, Dobbelstein S, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121(14):2618-2626.
doi pubmed - Franco S, Geng X, Korostyshevskiy V, Karp JE, Lai C. Systematic review and meta-analysis: Prognostic impact of time from diagnosis to treatment in patients with acute myeloid leukemia. Cancer. 2023;129(19):2975-2985.
doi pubmed - Waggoner M, Katsetos J, Thomas E, Galinsky I, Fox H. Practical management of the venetoclax-treated patient in chronic lymphocytic leukemia and acute myeloid leukemia. J Adv Pract Oncol. 2022;13(4):400-415.
doi pubmed - Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125(21):3246-3252.
doi pubmed - Bewersdorf JP, Zeidan AM. Hyperleukocytosis and leukostasis in acute myeloid leukemia: can a better understanding of the underlying molecular pathophysiology lead to novel treatments? Cells. 2020;9(10):2310.
doi pubmed - Marbello L, Ricci F, Nosari AM, Turrini M, Nador G, Nichelatti M, Tedeschi A, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia: a single-center retrospective study and review of literature. Leuk Res. 2008;32(8):1221-1227.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.