| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 000, Number 000, April 2025, pages 000-000
Inflammatory Cytokines in Association With High Fetal Hemoglobin Level Reduce the Episodes of Vaso-Occlusive Crisis in Sickle Cell Patients
Sujata Dixita, b, d, Arundhuti Dasa, d, Priyanka Samalc, Sonali Sandeeptaa, b, Swati Sudeshna Panigrahia, Hitesh Kumar Jaina, Subhojeet Biswasa, b, Mrutyunjay Suarb, Sanghamitra Patia, Manoranjan Ranjita, e, Madhusmita Bala, e
aDepartment of Immunology, Indian Council of Medical Research (ICMR)-Regional Medical Research Centre, Bhubaneswar 751023, Odisha, India
bSchool of Biotechnology, Kalinga Institute of Industrial Technology (KIIT) University, Bhubaneswar 751024, Odisha, India
cDepartment of Haematology, Institute of Medical Sciences (IMS) & SUM Hospital, Siksha ‘O’ Anusandhan (SOA) University, Bhubaneswar 751003, Odisha, India
dThese authors contributed equally to this work.
eCorresponding Author: Madhusmita Bal and Manoranjan Ranjit, ICMR-Regional Medical Research Centre, Bhubaneswar 751023, Indiaand
Manuscript submitted January 8, 2025, accepted March 21, 2025, published online April 14, 2025
Short title: Role of Cytokine and Hb F in SCD Patients
doi: https://doi.org/10.14740/jh1353
| Abstract | ▴Top |
Background: Vaso-occlusive crisis (VOC), a common clinical manifestation of sickle cell disease (SCD), is mediated by a series of inflammatory responses. Conversely, a high fetal hemoglobin (Hb F) level is a known factor that reduces the severity of clinical presentations associated with SCD. Since the association between cytokine profiles and high Hb F levels in SCD is not well studied, this study aims to investigate the interaction between cytokines and Hb F levels in SCD patients from Odisha, India.
Methods: A total of 276 patients with SCD were recruited for the study, including 180 in steady state and 90 in crisis state. Additionally, 117 individuals with sickle cell trait (SCT) and 128 healthy controls were included for comparison.
Results: The study revealed that the total white blood cell count and interleukin (IL)-6 levels were significantly higher in crisis state SCD patients, while the red cell distribution width, IL-10, and Hb F level were significantly higher in steady state SCD patients. Most importantly, the logistic regression analysis showed that the interaction of Hb F with the cytokines IL-10, IL-1β, and IL-17 provided three times greater protection from crisis in SCD patients.
Conclusion: The significance of these preliminary findings has been discussed in terms of prognostic markers and supplements to increase the efficacy of hydroxyurea used to enhance Hb F production.
Keywords: Cytokine; Sickle cell disease; VOC; Fetal hemoglobin; IL-6
| Introduction | ▴Top |
Vaso-occlusive crisis (VOC) is one of the most common clinical manifestations of sickle cell disease (SCD) and accounts for approximately 95% of hospitalizations related to this condition [1]. Most often it also leads to the development of other complications like acute chest syndrome (ACS), priapism, and hepatic/splenic sequestration. Consequently, there is a decrease in the health-related quality of life of the patients [2, 3]. VOC is a complex process triggered by the interactions between sickled red blood cells (RBCs) and the vascular endothelium, adherent leukocytes, and platelets in small blood vessels. The polymerization of sickled RBC causes a decrease in their flexibility and microvascular occlusion which involves complex interactions among leukocytes, the endothelium, and RBCs, ultimately causing tissue ischemia. These interactions are known to be mediated by cytokines secreted by T cells as well as adhesion molecules, and consequently, the immune response is implicated in the initiation and development of the sickle cell crisis [4]. Since sickle cell anemia is a chronic inflammatory disease, research has focused on the mechanisms involved in the inflammatory processes of sickle cell anemia so as to develop targeted and effective therapies to improve the quality of life [5].
India is estimated to have the second-highest burden of SCD patients after Nigeria [6], characterized by high levels of fetal hemoglobin (Hb F), varying frequencies of alpha thalassemia, and a distinct pathophysiology [7]. Identifying inflammatory factor(s)/cytokine (s) that mediate the susceptibility of individuals along with high Hb F, the most powerful modifier of disease severity in SCD [8, 9], can help in identifying new predictive markers and interventions against SCD severity.
Therefore, during this study, we have tried to study the association between cytokine profiles and high Hb F levels in order to understand the mechanisms that modulate disease severity in SCD with an aim to develop novel therapies that target inflammatory pathways within this population.
| Materials and Methods | ▴Top |
Patients presenting to the Outpatient Department or admitted to the Hematology Department of Institute of Medical Sciences (IMS) & SUM Hospital, tertiary care referral hospitals in Odisha, were clinically examined for SCD and suspected cases were referred to the ICMR-Regional Medical Research Centre for confirmatory laboratory diagnosis from April 20 to May 2023. SCD patients, less than 1 year old, had recently received blood transfusions, or were pregnant at the time of sample collection, were excluded from the study.
Ethical clearance
The study was approved by the Institutional Ethical Committee of ICMR-Regional Medical Research Centre and the Institute of Medical Sciences & SUM Hospital, Bhubaneswar (ICMR-RMRC/IHEC-2022/104, dated 09/03/2022). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Written consent was taken from all the participants before enrolling in this study.
Variable
Based on clinical severity, all SCD patients were categorized into two groups: group I - steady state and group II - crisis state. Steady state was defined as a period with no history of acute painful episodes, no history of underlying illness for the past 4 weeks, no history of blood transfusion in the previous 3 months, and no treatment with medications such as antibiotics, while crisis is characterized by bone and joint pain, multiple sites of discomfort, the requirement for analgesics, and potential hospitalization [2]. Demographic information, clinical history, medication history, blood transfusion records, and hospitalization details were also collected from participants.
Laboratory investigations
After obtaining informed consent, 5 mL of venous whole blood was collected from the subjects under aseptic conditions and equally divided into ethylenediaminetetraacetic acid (EDTA) tubes (BD Vacutainer® EDTA) and gel separator tubes (BD SST® II Advance). Whole blood in EDTA tubes was used for the acquisition of hematological data for RBCs, white blood cells (WBCs), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet/thrombocytes (THR), mean corpuscular volume (MCV), hematocrit (HCT), hemoglobin (Hb), and red cell distribution width (RDW) using an automatic hematological counter (Genrui KT-6400, China). Serum was obtained from the gel separator tubes after centrifugation at 2,000 × g at 4 °C for 2 min and stored at -20 °C for further use.
Diagnosis of hemoglobinopathy
The diagnosis of sickle cell was performed using cation-exchange high-performance liquid chromatography (HPLC; Bio-Rad VARIANT-II Haemoglobin Testing System), utilizing the β-thalassemia short program.
Cytokine analysis
Serum cytokine levels were measured using commercially available human enzyme-linked immunosorbent assay (ELISA) kits (Merck Life Science Pvt. Ltd; interleukin (IL)-6: catalog# RAB0306, IL-17: catalog# RAB0262, interferon (IFN)-γ: catalog# RAB0223, IL-10: catalog# RAB0244, and IL-1β: catalog# RAB0273 and Diaclone SAS, Besancon, France; tumor necrosis factor (TNF)-α: catalog# 950.090.096 and IL-8: catalog# 950.050.096). The sensitivity of the assay was 80 pg/mL for IL-17, 3 pg/mL for IL-6, 15 pg/mL for IFN-γ, 8 pg/mL for TNF-α, 12.3 pg/mL for IL-8, 1 pg/mL for IL-10, and 0.3 pg/mL for IL-1β. The assay was carried out according to the manufacturer’s instructions. Absorbance was measured at 450 nm and a linear standard curve was generated by plotting the average absorbance of each standard on the vertical axis versus the corresponding standard concentration on the horizontal axis. The level of cytokines in each sample was determined by extrapolating optical density (OD) values against standard concentration using the standard curve by using Graph Pad Prism software version 6.01 (La Jolla, CA, USA).
Statistical analysis
Categorical variables were reported as numbers and percentages, while continuous variables were reported as mean ± standard deviation (SD). Mann-Whitney test was used to analyze the difference between two groups of unpaired data using Graph Pad Prism software version 6.01 (La Jolla, CA, USA) and the regression analysis was done using STATA 14. P-values of < 0.05 were considered statistically significant.
| Results | ▴Top |
A total of 521 individuals participated in the study, comprising 260 (49.90%) males and 261 (50.09%) females, with a mean age of 26.69 years. Maximum number of cases belonged to the age group of 17 - 27 years, followed by 6 - 16 years (27.63%), 28 - 38 years (20.15%), 39 - 49 years (13.05%), 49 - 59 years (6.90%), and 59 - 65 (2.87%) (Table 1). Out of the 521 participants, 276 were SCD cases (145 males and 131 females) with a mean age of 24.3 years (range 1 - 65 years), 117 were SCT cases (54 males and 63 females) with a mean age of 31.8 years (range 1.5 - 58 years), and 128 were healthy controls (61 males and 67 females) with a mean age of 28.2 years (range 4 - 60 years). Out of 276 SCD cases recruited in the study, 96 (34.8%) were in crisis state and 180 (65.2%) were in steady state.
 Click to view | Table 1. Demographic Data of All Participants |
Hematological parameters in steady and crisis state SCD patients
The results of the hematological analysis (Table 2) showed that the WBC count was significantly higher in crisis state SCD patients compared to steady state SCD patients (P > 0.001), individuals with SCT (P > 0.05), and healthy control group (P > 0.05). The MCV was highest in steady state patients, followed by crisis state patients, healthy controls, and SCT cases. The differences were statistically significant between crisis and steady state patients (P > 0.001), between healthy control group and steady state patients (P > 0.001), and between SCT and steady state patients (P > 0.001). The MCH value was high in crisis and steady state patients when compared to SCT and healthy control groups. However, the difference was statistically significant only when comparing SCT with crisis state (P > 0.05) and steady state patients (P > 0.05). Similarly, MCHC values were higher in crisis state patients, but the difference was statistically significant only when comparing crisis with steady patients (P > 0.05) and between crisis patients and individuals with SCT (P > 0.05). Hb F level was higher in both steady state and crisis state SCD patients compared to SCT individuals and healthy controls. On the other hand, hematological parameters like RBC and HCT are high among SCT and health control groups when compared to steady state and crisis state SCD groups and the difference was statistically significant between all comparison groups, namely, crisis state and steady state patients, healthy control vs. crisis state patients, healthy control vs. steady patients, SCT vs. steady patients, SCT vs. crisis patients, and healthy control vs. SCT patients.
 Click to view | Table 2. Hematological Profile of Sickle Cell Patients in Steady State and Crisis State, SCT Group, and Healthy Control Group |
Overall, it was observed that WBC count was significantly higher in crisis patients when compared to steady patients, SCT individuals, and healthy control group. Additionally, RBC was lower in both steady and crisis SCD patients when compared to SCT and healthy control groups. HCT count was lower in crisis SCD patients compared to steady patients, SCT, and healthy control groups (Table 2).
Cytokine analysis
The IL-6, IL-17, IL-8, TNF-α, IFN- γ, IL-10, and IL-1β serum cytokine levels were measured in 43 crisis state patients and 130 steady state patients and compared with healthy controls. The Mann-Whitney test was employed to assess the association of aforementioned serum cytokine levels among different groups as seen in Figure 1. The expression of IL-6 was significantly higher in crisis state patients compared to healthy controls, but the same was not observed in the case of steady state. However, the level of IL-6 was significantly higher (P = 0.0013) in crisis state patients compared to steady state patients. Though IL-8 was significantly higher in both crisis state patients (P = 0.0001) and steady state patients (P < 0.0001) compared to healthy controls, no difference existed in the mean between the two SCD subgroups (steady and crisis). The levels of TNF-α and IL-1β were higher in SCD (both crisis and steady) cases compared to healthy controls, but no significant difference existed between the groups. The level of IL-17 was the same in both SCD cases and healthy ones. The expression of regulatory cytokine IL-10 was significantly higher in steady SCD cases (P = 0.033) compared to crisis and healthy ones.
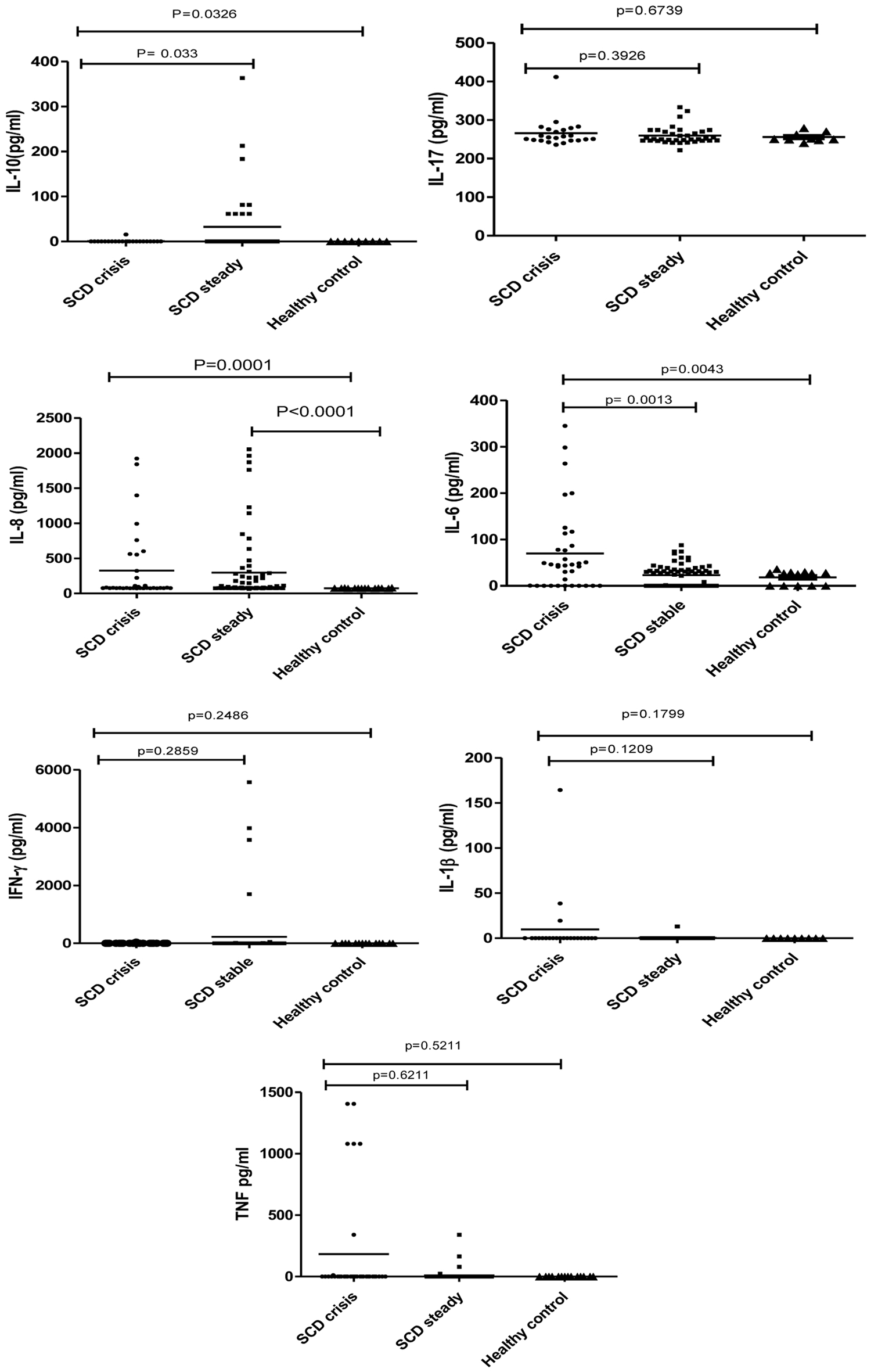 Click for large image | Figure 1. Association between cytokines and sickle cell disease patients in steady and crisis state, and healthy controls. |
Logistic regression analysis
Logistic regression analysis was applied to test the influence of cytokines and Hb F level on crisis state and steady state in aforementioned 173 SCD patients (43 crisis state patients and 130 steady crisis state patients). Multivariate logistic regression model (Table 3) showed that there was a possible interaction between Hb F and cytokines IL-17, IL-10, and IL-1β. The unadjusted beta coefficient of Hb F level against SCD crisis status changed from -0.050193 (0.113625, 0.0132385, P = 0.121) to -0.130237 (0.2307774, 0.0296959, P = 0.011) when interacting with IL-17 (model 1). This interaction significantly improved the role of Hb F by decreasing SCD crisis from 4.9% to 12.2%. Similarly, the unadjusted beta coefficient of Hb F level against SCD crisis status changed from -0.052174 (0.113625, 0.0132385, P = 0.121) to -0.132508 (0.0302486, 0.875896, P = 0.011) when interacting with IL-10 (model 2) and this interaction again significantly decreased SCD crisis from 4.9% to 12.4%. Along the same lines, the unadjusted beta coefficient of the Hb F level against SCD crisis status changed from -0.050193 (0.113625, 0.0132385, P = 0.121) to -0.130346 (-0.234307, -0.0263852, P = 0.014) when interacting with IL-1β (model 3). Like the observation in the previous two models, this interaction also decreased the chances of SCD crisis from 4.9% to 12.2%. Table 4 shows the results of logistic regression analysis examining the relationships between IL-6, IL-17, IL-8, TNF-α, IFN-γ, IL-10, IL-1β, and Hb F individually with SCD crisis state and steady state.
 Click to view | Table 3. Logistic Regression Models Showing Interaction Between Hb F and Cytokines IL-17, IL-10, and IL-β in Crisis and Steady SCD Patients |
 Click to view | Table 4. Logistic Regression Analysis of Cytokines, Hb F Level With Crisis and Steady SCD Patients |
| Discussion | ▴Top |
Hematological parameters and cytokine levels in serum seem to be useful predictors of the disease severity in SCD. In the present study, assessment of plasma cytokines such as IL-6, IL-10, IL-8, IL-17, TNF-α, IFN-γ, and IL-1β in SCD patients showed a significant elevation of IL-6 level in crisis state and IL-10 in steady state, but no difference was observed in IL-17, TNF-α, IFN-γ, and IL-1β levels in steady and crisis states, emphasizing the possible role of IL-6 in the development of VOC and protective role of IL-10 against disease severity. To the best of our knowledge, this is the first study among the Indian SCD population that corroborates with the findings of the studies conducted in Brazil [10], Bahrain [11], and Egypt [12].
The possible mechanism of elevation of IL-6 level might be due to the effects of by-products of hemolysis of sickle cell RBCs, particularly microparticles (MPs) and damage-associated molecular patterns (DAMPs) [13]. Because, in other such studies, it has been shown that the MPs of platelets and leukocytes origin and DAMPs increase the production of IL-6 [14, 15] and upregulation of IL-6 in turn stimulates endothelial MPs to induce endothelial dysfunction. It is known that endothelial dysfunction leads to a loss of vascular integrity, expression of leukocyte adhesion molecules, change in the surface phenotype from antithrombotic to prothrombotic, excessive cytokine production, and ultimately increasing adhesion molecules like endothelial intercellular adhesion molecule 1 (ICAM-1) and neutrophil adhesion [16]. Several studies have also shown that MPs in circulation have been linked to the regulation of endothelial function in a range of vascular pathologies-related disorders like cardiovascular disease, ischemic coronary artery disease [17], diabetes [18], and infectious diseases such as cerebral malaria [19]. Hence, this could be one of the probable mechanisms behind the observed increased level of IL-6 production and development of crisis in the studied patients. On the other hand, IL-10, considered as a regulatory cytokine, has a broad spectrum of immunomodulatory and anti-inflammatory activity in various cases of inflammation and infection. Significantly high IL-10 levels among steady state SCD patients in the present study align with the observation made by others, who found that IL-10 levels were reduced in sickle cell children with osteomyelitis [11]. However, the increased production and activation of B cells in sickle cell anemia patients through various receptors (B-cell receptor (BCR), CD40, and Toll-like receptors (TLRs)) has been found to trigger intracellular signaling pathways like phosphatidylinositol 3-kinase (PI3K)-Akt and nuclear factor-kappa B (NF-κB) and promote the expression of transcription factors that drive IL-10 production [20-22]. The inverse relationship between the expression of major inflammatory cytokines IL-6 and IL-10 in the crisis stage of SCD patients in the present study may be attributed to the immune-inhibitory role of IL-10, which primarily depends on antigen-presenting cells and the production of Th1 cytokines. Although our study did not reveal any differences in the levels of IL-8, IL-17, IL-1β, and IFN-γ, studies conducted in Iran and Oman have shown a positive correlation between increased levels of IL-8 and IL-17 and the crisis state [23, 24]. This may be due to the association between various β-globin haplotypes and cytokine polymorphisms that regulates the level of cytokine expression [25, 26].
Similarly, we have found a significantly higher WBC count in the crisis state, compared to the steady state and the normal control group as reported earlier in Ghana and the Nigerian population [9, 27-29]. The high WBC count, a marker of the chronic inflammatory state, may result from functional hyposplenism and asplenia in the SCD population, triggered by the stimulus for erythropoiesis or subclinical infection [30], and during the crisis state of SCD, WBC might be playing a significant role in tissue damage, inflammation, and ultimately vaso-occlusion by stimulating the production of vascular endothelial ligands for the blood cell adhesion molecules [31]. Similarly, high level of Hb F among Indian SCD patients compared to the African population represents a distinctive hematological feature that has been found to be associated with less severe presentation of various clinical manifestations [32, 33]. High level of Hb F increases the flexibility of RBCs, thus preventing hemolysis and subsequent MP generation, and might be endothelial activation. During regression analysis, it was revealed that, independently, IL-17, IL-10, IL-1β, and the Hb F levels do not influence SCD crisis status significantly. The analysis showed that in the presence of Hb F, the chances of a crisis event were reduced by 4%; however, interaction of Hb F with cytokines IL-10, IL-1β, and IL-17, reduces the chances of SCD crisis events by three times (12%). Although the role of cytokines in increasing the function of Hb F is not known, yet an ex vivo study conducted on adult erythroid cells showed that cytokine-mediated signal transduction increases the production of Hb F by mediating epigenetic modifications in the globin genes [34]. With this backdrop, we hypothesize (which will need further research, using cell culture method to be proven) that increased Hb F levels, probably induced by certain cytokines, increase the flexibility of RBCs. This increased flexibility may reduce endothelial dysfunction by decreasing the production of MPs and inflammatory cytokines, thereby mitigating the subsequent cascade of inflammatory responses. Consequently, this mechanism could protect patients from crisis.
In conclusion, the present study reveals a significant association between WBC count and IL-6 levels with the development of VOC. These two variables can be used as predictive markers. Conversely, a significant reduction in the incidence of crisis state with high Hb F levels, along with IL-10, IL-1β, and IL-17, indicates that these cytokines can be considered as complimentary therapeutic agents to enhance the effectiveness of hydroxyurea. However, further extensive studies are needed.
Acknowledgments
The authors thankfully acknowledge the Indian Council of Medical Research for providing all necessary laboratory facilities for analyzing the samples. The authors also express their gratefulness to all the individuals who have participated voluntarily in this research project. Additionally, we thank Dr. Ambarish Dutta and Dr. Jyoti Ghoshal for their discussions during analysis of the data.
Financial Disclosure
No extramural funding was received for the project.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All subjects provided written informed consent.
Author Contributions
MRR, MB, and SD developed the research proposal and manuscript preparation. SD and AD executed the project, and were involved in sample collection and data analysis. HKJ and SB were involved in field survey, data, and sample collection. SD, SS, and SSP were involved in laboratory studies. PS analyzed the clinical data. MS helped in manuscript preparation and writing. SP provided intellectual and administrative support during field studies and revised the manuscript. All authors have read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACS: acute chest syndrome; EDTA: ethylenediaminetetraacetic acid; Hb F: fetal hemoglobin; HCT: hematocrit; IFN: interferon; IL: interleukin; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; OD: optical density; RBC: red blood cell; RDW: red cell distribution width; SCD: sickle cell disease; SCT: sickle cell trait; SD: standard deviation; TNF: tumor necrosis factor; VOC: vaso-occlusive crisis; WBC: white blood cell
| References | ▴Top |
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79(1):17-25.
doi pubmed - Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647-3656.
doi pubmed - van Tuijn CF, van Beers EJ, Schnog JJ, Biemond BJ. Pain rate and social circumstances rather than cumulative organ damage determine the quality of life in adults with sickle cell disease. Am J Hematol. 2010;85(7):532-535.
doi pubmed - Musa BO, Onyemelukwe GC, Hambolu JO, Mamman AI, Isa AH. Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis. Clin Vaccine Immunol. 2010;17(4):602-608.
doi pubmed - Chies JA, Nardi NB. Sickle cell disease: a chronic inflammatory condition. Med Hypotheses. 2001;57(1):46-50.
doi pubmed - Hockham C, Bhatt S, Colah R, Mukherjee MB, Penman BS, Gupta S, Piel FB. The spatial epidemiology of sickle-cell anaemia in India. Sci Rep. 2018;8(1):17685.
doi pubmed - Serjeant GR. Sickle Cell Disease: Thoughts for India from the Jamaican cohort study. Front Med (Lausanne). 2021;8:745189.
doi pubmed - Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P, Chui DH, et al. Fetal hemoglobin in sickle cell anemia. Blood. 2011;118(1):19-27.
doi pubmed - Antwi-Boasiako C, Frimpong E, Ababio GK, Dzudzor B, Ekem I, Gyan B, Sodzi-Tettey NA, et al. Sickle cell disease: reappraisal of the role of Foetal Haemoglobin levels in the frequency of Vaso-occlusive crisis. Ghana Med J. 2015;49(2):102-106.
doi pubmed - Domingos IF, Pereira-Martins DA, Sobreira M, Oliveira RTD, Alagbe AE, Lanaro C, Albuquerque DM, et al. High levels of proinflammatory cytokines IL-6 and IL-8 are associated with a poor clinical outcome in sickle cell anemia. Ann Hematol. 2020;99(5):947-953.
doi pubmed - Sarray S, Saleh LR, Lisa Saldanha F, Al-Habboubi HH, Mahdi N, Almawi WY. Serum IL-6, IL-10, and TNFalpha levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine. 2015;72(1):43-47.
doi pubmed - Elammary Y, Sewelam N, Al-Wakeel H, El-Ghamrawy M, Zayed S. Interleukin-1β and interleukin-6 gene polymorphisms in Egyptian sickle cell disease patients. Egyptian Pediatric Association Gazette. 2020;68:1-7.
- Mendonca R, Silveira AA, Conran N. Red cell DAMPs and inflammation. Inflamm Res. 2016;65(9):665-678.
doi pubmed - Cognasse F, Nguyen KA, Damien P, McNicol A, Pozzetto B, Hamzeh-Cognasse H, Garraud O. The inflammatory role of platelets via their TLRs and Siglec receptors. Front Immunol. 2015;6:83.
doi pubmed - Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295.
doi pubmed - Garnier Y, Ferdinand S, Garnier M, Cita KC, Hierso R, Claes A, Connes P, et al. Plasma microparticles of sickle patients during crisis or taking hydroxyurea modify endothelium inflammatory properties. Blood. 2020;136(2):247-256.
doi pubmed - Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat ML, Boilard E, et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost. 2017;117(7):1296-1316.
doi pubmed - Feng B, Chen Y, Luo Y, Chen M, Li X, Ni Y. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2010;208(1):264-269.
doi pubmed - Sahu U, Sahoo PK, Kar SK, Mohapatra BN, Ranjit M. Association of TNF level with production of circulating cellular microparticles during clinical manifestation of human cerebral malaria. Hum Immunol. 2013;74(6):713-721.
doi pubmed - Bao W, Zhong H, Manwani D, Vasovic L, Uehlinger J, Lee MT, Sheth S, et al. Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. Am J Hematol. 2013;88(9):736-740.
doi pubmed - Kaaba SA, al-Harbi SA. Reduced levels of CD2+ cells and T-cell subsets in patients with sickle cell anaemia. Immunol Lett. 1993;37(1):77-81.
doi pubmed - Young RM, Shaffer AL, 3rd, Phelan JD, Staudt LM. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):77-85.
doi pubmed - Keikhaei B, Mohseni AR, Norouzirad R, Alinejadi M, Ghanbari S, Shiravi F, Solgi G. Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. Eur Cytokine Netw. 2013;24(1):45-52.
doi pubmed - Pathare A, Al Kindi S, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D. Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004;77(4):323-328.
doi pubmed - Bandeira IC, Rocha LB, Barbosa MC, Elias DB, Querioz JA, Freitas MV, Goncalves RP. Chronic inflammatory state in sickle cell anemia patients is associated with HBB(*)S haplotype. Cytokine. 2014;65(2):217-221.
doi pubmed - Olenscki Gilli SC, Pericole FV, Benites BD, Sippert EA, Castilho LM, Addas-Carvalho M, Olalla Saad ST. Cytokine polymorphisms in sickle cell disease and the relationship with cytokine expression. Exp Hematol. 2016;44(7):583-589.
doi pubmed - Omoti CE. Haematological values in sickle cell anaemia in steady state and during vaso-occlusive crisis in Benin City, Nigeria. Annals of African Medicine. 2005;4(2):62-67.
- Mombo LE, Mabioko-Mbembo G, Bisseye C, Mbacky K, Thiam F, Edou A. Haematological values in steady-state sickle cell anaemia patients and matched heamoglobin AA Controls in a Rural Area of Eastern Gabon. Niger Postgrad Med J. 2019;26(1):13-17.
doi pubmed - Abubakar Y, Ahmad HR, Faruk JA. Hematological parameters of children with sickle cell anemia in steady and crisis states in Zaria, Nigeria. Annals of Tropical Pathology. 2019;10(2):122-125.
- Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. 2002;24(2):81-88.
doi pubmed - Yousif TYE. Impact of abnormal leukocyte count in the pathophysiology of sickle cell anemia. J Blood Med. 2022;13:673-679.
doi pubmed - Colah RB, Mukherjee MB, Martin S, Ghosh K. Sickle cell disease in tribal populations in India. Indian J Med Res. 2015;141(5):509-515.
doi pubmed - Jit BP, Mohanty PK, Purohit P, Das K, Patel S, Meher S, Mohanty JR, et al. Association of fetal hemoglobin level with frequency of acute pain episodes in sickle cell disease (HbS-only phenotype) patients. Blood Cells Mol Dis. 2019;75:30-34.
doi pubmed - Sripichai O, Kiefer CM, Bhanu NV, Tanno T, Noh SJ, Goh SH, Russell JE, et al. Cytokine-mediated increases in fetal hemoglobin are associated with globin gene histone modification and transcription factor reprogramming. Blood. 2009;114(11):2299-2306.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.