| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 000, Number 000, April 2025, pages 000-000
Ruxolitinib Is an Effective Therapy for Ciltacabtagene Autoleucel-Associated Parkinsonism in Multiple Myeloma
Baldeep Wirka, c, Jin Limb
aCellular Immunotherapies and Transplant Program, Massey Comprehensive Cancer Center, Virginia Commonwealth University, Richmond, VA 23219, USA
bDepartment of Radiology, Virginia Commonwealth University, Richmond, VA 23219, USA
cCorresponding Author: Baldeep Wirk, Cellular Immunotherapies and Transplant Program, Massey Comprehensive Cancer Center, Virginia Commonwealth University, Richmond, VA 23219, USA
Manuscript submitted February 13, 2025, accepted April 11, 2025, published online April 22, 2025
Short title: Ruxolitinib Reverses Cilta-Cel-Associated Parkinsonism
doi: https://doi.org/10.14740/jh2046
| Abstract | ▴Top |
After ciltacabtagene autoleucel (cilta-cel) in multiple myeloma, 5% of patients can develop parkinsonism, with a high fatality rate. The pathogenesis and optimal therapy of parkinsonism from B-cell maturation antigen chimeric antigen receptor T-cell (CAR T-cell) therapy are unknown. Parkinson’s disease occurs from the loss of dopaminergic neurons in the substantia nigra. However, in cilta-cel-associated parkinsonism, dopamine transporter imaging is normal, rendering traditional agents such as carbidopa/levodopa ineffective. Thus, the pathogenesis of cilta-cel-associated parkinsonism and Parkinson’s disease is distinct. As CAR T-cell therapy for multiple myeloma is expanding and moving to earlier lines, the need to optimize therapy for parkinsonism, a potentially life-threatening complication, becomes more urgent. This report presents the first documented cases of two patients with immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome and cilta-cel-associated parkinsonism, effectively treated with ruxolitinib.
Keywords: Parkinsonism; CAR T-cell therapy; Cilta-cel; Multiple myeloma; Ruxolitinib
| Introduction | ▴Top |
Movement and neurocognitive treatment-emergent adverse events (MNTs) occur with B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T-cell therapy for multiple myeloma (MM), such as ciltacabtagene autoleucel (cilta-cel) and idecabtagene vicleucel [1, 2]. In the CARTITUDE-1 study of cilta-cel in relapsed/refractory MM, 5% of patients developed MNTs with parkinsonism, which can be fatal [3, 4]. Three of five parkinsonism patients died from neurotoxicity or infection [4]. In a real-world experience of cilta-cel in 236 relapsed/refractory MM patients, five patients developed parkinsonism. Only one patient had partial improvement in parkinsonism, whereas the remaining four patients died [5]. In addition, five patients developed immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS), a hyperinflammatory syndrome characterized by hyperferritinemia, pancytopenia, coagulopathy, transaminitis, and organ failure [5].
The pathogenesis and optimal therapy of cilta-cel-associated-parkinsonism are unknown, with sparse information in the literature. Parkinson’s disease occurs from the loss of dopaminergic neurons in the substantia nigra, as demonstrated by abnormal activation of dopamine transporters, often seen on I-123 ioflupane dopamine transporter imaging. In contrast, dopamine transporter imaging is normal in cilta-cel-associated parkinsonism, rendering traditional agents such as carbidopa/levodopa ineffective [3, 4]. Furthermore, autopsies of two patients with MNTs in CARTITUDE-1 showed an intact substantia nigra, pointing to a distinct pathogenesis of cilta-cel-associated parkinsonism from Parkinson’s disease [4].
MNTs occur after recovery from cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity (ICANS) with a median onset on day 27 (range 14 - 108) post cilta-cel infusion [4]. Therapies, such as corticosteroids, intrathecal chemotherapy, systemic chemotherapy (cyclophosphamide), and anakinra, have been tried with minimal responses [4].
Using immunosuppressive therapy, such as cyclophosphamide, to rapidly clear CAR T cells showed mixed responses with opportunistic infections and even death in two of the three cases [1-3]. A CARTITUDE-1 patient who developed parkinsonism received intravenous (IV) cyclophosphamide. The patient died of multi-organ failure on day 162 after cilta-cel [3]. At autopsy, some neurons and astrocytes in the caudate nucleus and frontal cortex had BCMA expression, alluding to an on-target off-tumor effect of cilta-cel [3, 4]. In contrast, another study showed undetectable immunoreactivity to BCMA protein in normal adult brains [6].
As CAR T-cell therapy for MM is expanding and moving to earlier lines, the need to optimize therapy for parkinsonism, a potentially life-threatening complication, becomes more urgent. This report presents the first documented cases of two patients with IEC-HS and cilta-cel-associated parkinsonism, effectively treated with ruxolitinib.
| Case Reports | ▴Top |
Case 1
A 53-year-old female with Revised International Staging System (R-ISS) I kappa light chain MM, 46XX, +1q, del 17p13, and DNMT3A mutation (variant allele frequency 6.79%) became penta-refractory to lenalidomide, bortezomib, carfilzomib, pomalidomide, and daratumumab over 3 years. She had no personal or family history of Parkinson’s disease. Before cilta-cel infusion, she had a low tumor burden (bone marrow clonal plasmacytosis 15%; serum free kappa light chain (SFKLC) 1,436 mg/L (normal 3.3 - 19.4 mg/L)). She had lymphodepletion with standard fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 daily for 3 days followed by cilta-cel 0.6 × 106/kg infusion on day 0. Day 0 absolute lymphocyte count (ALC) was 0.1 × 109/L (1.3 to 3.6 × 109/L). Day 6 post-infusion, two doses of tocilizumab resolved the grade 2 CRS. No ICANS occurred. Grade 2 IEC-HS developed on day 10: hyperferritinemia > 30,000 ng/mL (16 - 150 ng/mL) with baseline ferritin 451 ng/mL; transaminitis; hypofibrinogenemia 90 mg/dL (200 - 450 mg/dL); soluble interleukin-2 receptor (sIL-2R) 8,180 U/mL (223 - 770 U/mL); chemokine C-X-C motif ligand 9 (CXCL9) 57,240 pg/mL (< 647 pg/mL), and worsening pancytopenia (grade 4 neutropenia and thrombocytopenia). The maximum ALC occurred on day 12: 2.1 × 109/L. The CD4+ T-cell count was 64/mm3 (480 - 1,800) at day 14 and ALC was 1.3 × 109/L on day 21. The IEC-HS was treated with anakinra 100 mg IV every 8 h for 5 days, dexamethasone 10 mg IV every 8 h, and cryoprecipitate, with resolution of the transaminitis, but not the hypofibrinogenemia, hyperferritinemia, and pancytopenia.
On day 30, the patient developed hypomimia, hypophonia, cogwheel rigidity, micrographia, bradykinesia, inability to stand without assistance, and shuffling gait. She could not initiate a conversation but did give appropriate one-word answers (the immune effector cell encephalopathy (ICE) score remained 10; ICANS 0; CRS 0).
Brain magnetic resonance imaging (MRI) with/without contrast showed an increased T1 signal of the bilateral basal ganglia not seen on the MRI before cilta-cel infusion (Fig. 1a). The brain (18F) fluorodeoxyglucose-positron emission tomography (FDG-PET) showed hypometabolism of the caudate and frontal lobes (Fig. 2a, b) as demonstrated by statistically significant increased chromatic aberrations seen on voxel-based PET analysis when compared to a normalized population dataset. The electroencephalogram (EEG), carotid ultrasound, and brain dopamine transporter scan were normal (Fig. 1c). The cerebrospinal (CSF) cell counts, glucose, protein, cytology, meningoencephalitis, autoimmune, and paraneoplastic panels were normal. The serum viral, fungal, and parasitic panels were negative, including JC virus, human herpesvirus 6 (HHV6), parvovirus B19, and strongyloides. Neuro-oncology diagnosed cilta-cel-associated parkinsonism stage 4 by the Hoehn and Yahr scale [7]. The hyperferritinemia, pancytopenia, and hypofibrinogenemia persisted.
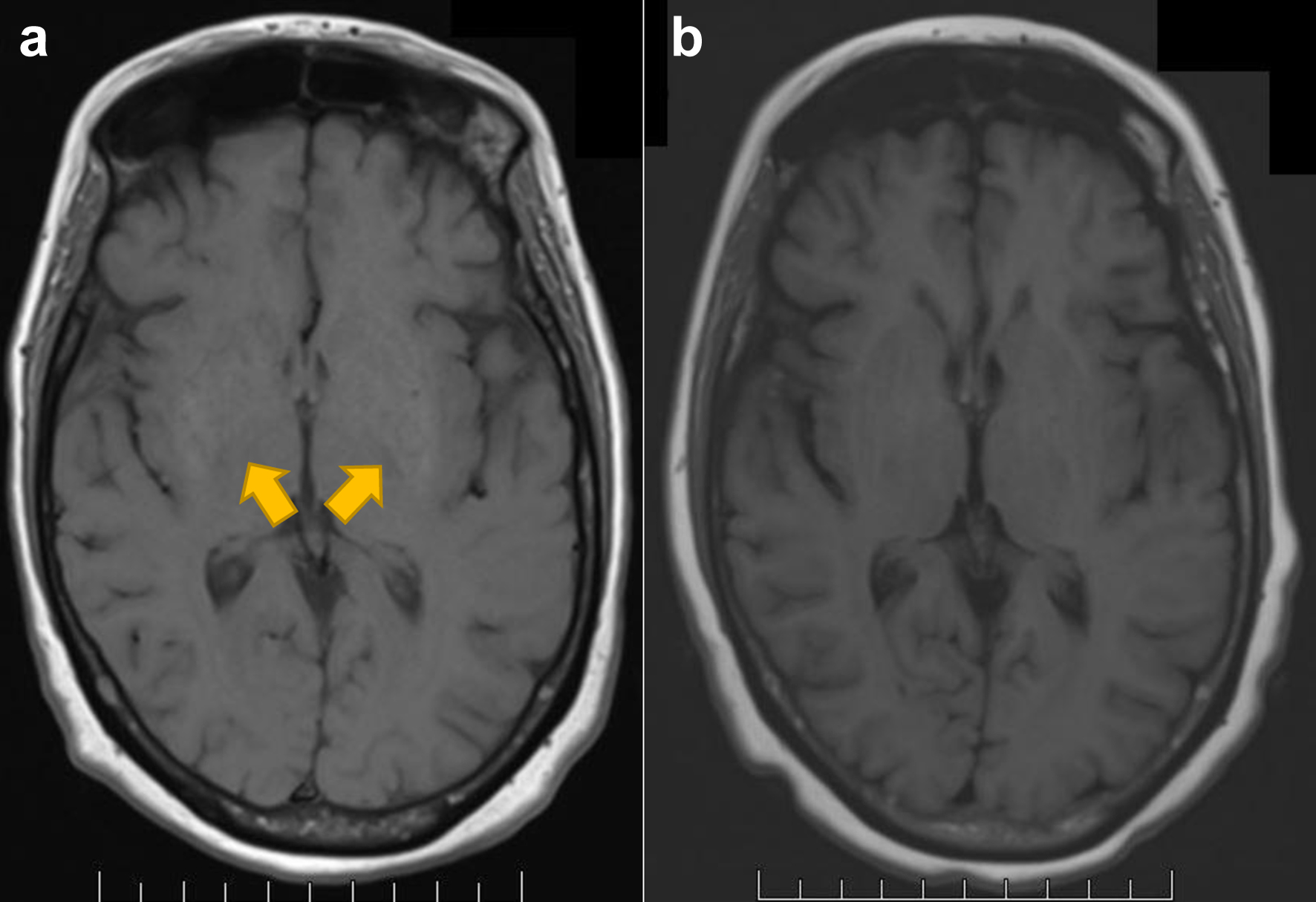 Click for large image | Figure 1. Structural imaging with MRI T1WI of the brain for case 1. (a) Increased T1 signal of the basal ganglia (arrowheads) at diagnosis, not seen before cilta-cell infusion. (b) Post-treatment scan with resolution of prior T1 hyperintense signal of basal ganglia. MRI: magnetic resonance imaging; T1WI: T1-weighted image. |
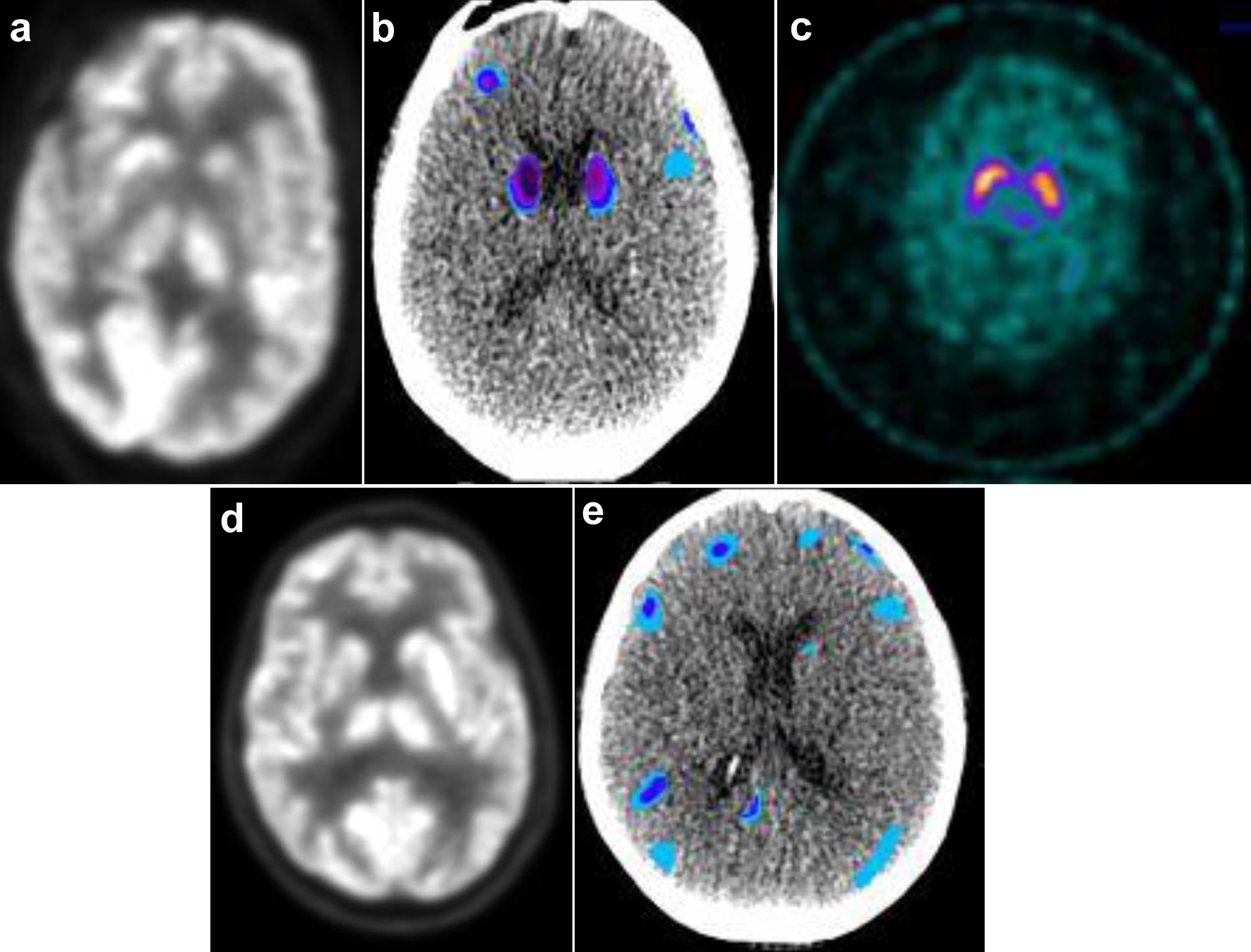 Click for large image | Figure 2. Functional imaging for case 1 (a-c) and case 2 (d, e). (a) PET AC and (b) voxel-based analysis of brain PET normalized to standard dataset using MIMneuro software show significant decreased metabolism in bilateral caudate and frontal lobe/gyri. (c) DAT SPECT scan demonstrates symmetric normal uptake of basal ganglia. (d) PET AC and (e) normalized voxel-based analysis of PET brain demonstrate significant decreased metabolism in bilateral frontal lobe/gyri. AC: attenuation correction; DAT: dopamine activated transporter; PET: positron emission tomography; SPECT: single photon emission computed tomography. |
On day 30, the patient received IV gammaglobulin 2 g/kg and methylprednisolone 1 g (for 3 days and tapered off by day 148) without improvement of the parkinsonism. Due to persistent IEC-HS, ruxolitinib 5 mg twice daily was given on day 95. In 3 weeks of starting ruxolitinib, the micrographia, cogwheel rigidity, bradykinesia, hypomimia, and hypophonia resolved. The patient could stand from recumbency and walk unassisted and speak spontaneously. On day 157, the brain MRI showed resolution of the hyperintense T1 signal in the basal ganglia (Fig. 1b). The pancytopenia resolved, and the ruxolitinib was increased to 10 mg twice daily on day 163. The fibrinogen and sIL-2R normalized, and the ferritin level improved to 782 ng/mL. The patient is doing well on ruxolitinib 10 mg twice daily without any opportunistic infections. She can conduct all activities of daily living (ADLs) independently. The patient achieved stringent complete remission, minimal residual disease negative by bone marrow ClonoSEQ and whole-body PET-CT at day 100, sustained 1 year after cilta-cel infusion at the time of this report.
Case 2
A 70-year-old male presented with IgAk MM R-ISS I with del 17p13, del 1p36, del 14q32, 46XY a year before cilta-cel therapy. He was refractory to daratumumab, lenalidomide, bortezomib, with dexamethasone and salvage pomalidomide and ixazomib. He had no personal or family history of Parkinson’s disease. He had low-burden disease (bone marrow plasmacytosis 40%, serum monoclonal IgAk 0.8 g/dL, and SFKLC 56.87 mg/L) before lymphodepletion with standard fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 daily for 3 days followed by cilta-cel 0.6 × 106/kg infusion on day 0. Day 0 ALC was 0.2 × 109/L.
On day 8 post-infusion, grade 1 CRS resolved with two doses of tocilizumab. The maximum ALC was 39.1 × 109/L on day 12. On day 17, the ALC was 7.6 × 109/L, and he presented with hypomimia, hypophonia, bradykinesia, cogwheel rigidity, shuffling gait, and micrographia. Further testing revealed grade 2 IEC-HS with hypofibrinogenemia 99 mg/dL; hyperferritinemia 9,258 ng/mL with baseline ferritin 265 ng/mL; transaminitis; sIL-2R 2,826 U/mL; CXCL9 57,240 pg/mL, and worsening pancytopenia (grade 3 neutropenia, anemia, and thrombocytopenia). ICE score was 10, CRS was 0, and ICANS was 0.
Brain MRI with/without contrast was normal. Brain FDG-PET showed hypometabolism of the bilateral frontal lobes (Fig. 2d, e) with statistically significant increased chromatic aberrations seen on normalized voxel-based PET analysis. The EEG and brain dopamine transporter scan were normal. The CSF cell counts, glucose, protein, cytology, meningoencephalitis, autoimmune, and paraneoplastic panels were unrevealing. The serum viral, fungal, and parasitic panels were unremarkable. Neuro-oncology diagnosed cilta-cel-associated parkinsonism stage 4 by the Hoehn and Yahr scale [7].
He began IV gammaglobulin 2 g/kg; IV methylprednisolone 1 g daily for 3 days, tapered off by day 67; IV anakinra 100 mg every 8 h for 7 days; and ruxolitinib 5 mg tablet twice daily. The IEC-HS resolved by day 50. On day 21, the ALC was 0.5 × 109/L. The bradykinesia, cogwheel rigidity, shuffling gait, micrographia, and hypophonia resolved by day 57 and the hypomimia by day 70. He tolerated the ruxolitinib well without any opportunistic infections. On day 165, the ruxolitinib was tapered to 5 mg daily without recurrence of the parkinsonism. He is conducting all ADLs independently. The patient has resumed driving a motor vehicle. The patient attained stringent complete remission, minimal residual disease negative by bone marrow ClonoSEQ and whole-body PET-CT at day 100, sustained 6 months after cilta-cel infusion at the time of this writing.
| Discussion | ▴Top |
ICANS occurred in 16% of patients in CARTITUDE-1, mostly grade 1 or 2, lasting a median of 4 days (1 - 12 days) [4]. However, for the 5% of patients with cilta-cel-associated parkinsonism, the recovery takes longer (median 70 days) in those that survive [4]. Cilta-cel-associated parkinsonism can be fatal. In the literature, risk factors for developing MNTs after cilta-cel therapy include high tumor burden (bone marrow clonal plasmacytosis > 80%; serum monoclonal spike ≥ 5 g/dL; and involved serum free light chain ≥ 5,000 mg/L) [4]. Despite having a low tumor burden (bone marrow plasmacytosis < 50%; serum M spike < 3 g/dL; and involved serum free light chain < 3,000 mg/L), our patients still developed cilta-cel-associated parkinsonism. Other predictors for developing MNTs include CD4+ T-cell count > 1,000/mm3 at day 14 and ALC > 3 × 109/L on day 21 post-infusion, which are surrogate markers for high cilta-cel expansion and persistence since measuring CAR T cells in the real-world clinical setting is challenging [4]. Only case 2 had lymphocytosis. CRS grade ≥ 2 is another predictive marker for developing MNTs, seen in case 1 but not case 2 [4]. Four of five parkinsonism patients in the CARTITUDE-1 study had prior ICANS, not seen in either of our patients.
The DNMT3A, TET2, and ASXL1 (DTA) mutations are linked with inflammation and greater neurotoxicity after CAR T-cell therapy in lymphoma [8]. Plasma interleukin-6 (IL-6) levels, grade ≥ 3 ICANS (58.9% vs. 25%, P = 0.02), and grade ≥ 3 CRS (17.7% vs. 4.2%, P = 0.08) incidences were higher in DTA-positive than in DTA-negative patients after CAR T-cell therapy [8]. Case 1 had a DNMT3A mutation, which may have accounted for more significant neurotoxicity after cilta-cel, although more studies are needed. Case 2 had no clonal hematopoiesis.
In other case reports of cilta-cel-associated parkinsonism, there was an increased T1 signal of the bilateral basal ganglia on brain MRI and hypometabolism of the caudate and frontal lobes on brain FDG-PET, as in our patients [1-3]. The T1 signal from the brain MRI was reversible after the parkinsonism improved in case 1. The dopamine transporter scan was normal in both patients, indicating a distinct pathophysiology from Parkinson’s disease.
Both patients developed grade 2 IEC-HS after CRS resolution, with hyperferritinemia, hypofibrinogenemia, transaminitis, and pancytopenia [9]. Neurological complications, such as ataxia, seizures, and MRI brain basal ganglia hyperintensity, occur in primary and secondary hemophagocytic lymphohistiocytosis [10]. However, the neurological manifestations of IEC-HS are unknown.
Ruxolitinib blocks signal transduction in the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway by inhibiting JAK1 and JAK2 and reduces the activity of proinflammatory cytokines, such as interferon (IFN)-gamma, interleukin-2 (IL-2), and IL-6, without impairing T-cell cytotoxicity in IEC-HS [11-13]. This is particularly significant as peak IL-6 and IFN-gamma levels were higher in patients with MNTs from cilta-cel, suggesting the promising potential of ruxolitinib in treating MNTs [4].
Ruxolitinib is also effective in hemophagocytic lymphohistiocytosis as frontline or salvage therapy by reducing the activity of INF-gamma, IL-2, and IL-6 [12-15]. We avoided high-dose cyclophosphamide since only one case report showed improvement in cilta-cel-associated parkinsonism, albeit with opportunistic infections [2]. Cyclophosphamide compounds the immunosuppression of CAR T-cell therapy, further increasing the risk for opportunistic infections [1, 3]. Indeed, infection is the primary cause of non-relapse mortality (50.9%) from CAR T-cell therapy [16]. Neither of our patients had opportunistic infections while on ruxolitinib.
Case 1 had ruxolitinib in the salvage setting for IEC-HS since neither anakinra, methylprednisolone, nor IV immunoglobulin improved the IEC-HS or the parkinsonism. Case 2 had frontline ruxolitinib. Importantly, neither patient had opportunistic infections. In both cases, there was resolution of the IEC-HS, and in parallel, the parkinsonism improved by 4 months in case 1 and 2 months in case 2, likely reflecting the use of ruxolitinib in the salvage setting in case 1 and frontline in case 2 (Table 1).
 Click to view | Table 1. Inflammatory Markers Before and After Ruxolitinib |
The neurological manifestations of IEC-HS are unknown. It is intriguing to postulate that parkinsonism is a neurological manifestation of IEC-HS. Or is IEC-HS a predictive factor for cilta-cel-associated parkinsonism? These are the first two patients reported who had IEC-HS concomitantly with parkinsonism from cilta-cel. Further studies are needed. In the meantime, in patients with cilta-cel-associated parkinsonism, it is essential to assess for IEC-HS, as both conditions can be life-threatening. Since precise predictive factors for developing cilta-cel-associated parkinsonism are unknown, it is imperative to monitor patients for at least 6 months post-cilta-cel infusion. Imaging can help in the interim to gauge cilta-cel-associated parkinsonism and recovery after immunotherapy.
In conclusion, ruxolitinib is a life-saving therapy for IEC-HS and cilta-cel-associated parkinsonism and should be further explored in clinical studies.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patients.
Author Contributions
Baldeep Wirk and Jin Lim wrote article and collected the data.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Karschnia P, Miller KC, Yee AJ, Rejeski K, Johnson PC, Raje N, Frigault MJ, et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood. 2023;142(14):1243-1248.
doi pubmed - Graham CE, Lee WH, Wiggin HR, Supper VM, Leick MB, Birocchi F, Yee AJ, et al. Chemotherapy-induced reversal of ciltacabtagene autoleucel-associated movement and neurocognitive toxicity. Blood. 2023;142(14):1248-1252.
doi pubmed - Van Oekelen O, Aleman A, Upadhyaya B, Schnakenberg S, Madduri D, Gavane S, Teruya-Feldstein J, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27(12):2099-2103.
doi pubmed - Cohen AD, Parekh S, Santomasso BD, Gallego Perez-Larraya J, van de Donk N, Arnulf B, Mateos MV, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12(2):32.
doi pubmed - Sidana S, Patel KK, Peres LC, Bansal R, Kocoglu MH, Shune L, Atrash S, et al. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood. 2025;145(1):85-97.
doi pubmed - Marella M, Yao X, Carreira V, Bustamante MF, Clark HB, Jackson CC, Zudaire E, et al. Comprehensive BCMA expression profiling in adult normal human brain suggests a low risk of on-target neurotoxicity in BCMA-targeting multiple myeloma therapy. J Histochem Cytochem. 2022;70(4):273-287.
doi pubmed - Muller J, Wenning GK, Jellinger K, McKee A, Poewe W, Litvan I. Progression of Hoehn and Yahr stages in Parkinsonian disorders: a clinicopathologic study. Neurology. 2000;55(6):888-891.
doi pubmed - Saini NY, Swoboda DM, Greenbaum U, Ma J, Patel RD, Devashish K, Das K, et al. Clonal hematopoiesis is associated with increased risk of severe neurotoxicity in axicabtagene ciloleucel therapy of large B-cell lymphoma. Blood Cancer Discov. 2022;3(5):385-393.
doi pubmed - Hines MR, Knight TE, McNerney KO, Leick MB, Jain T, Ahmed S, Frigault MJ, et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome. Transplant Cell Ther. 2023;29(7):438.e1-438.e16.
doi pubmed - He T, Zhong Y, Li H, Jiang F, Cai H, Yang H, Ouyang S, et al. Central nervous system involvement in adult-onset hemophagocytic lymphohistiocytosis secondary to lymphoma: a case presentation and literature analysis. Quant Imaging Med Surg. 2023;13(6):4032-4040.
doi pubmed - Das R, Guan P, Sprague L, Verbist K, Tedrick P, An QA, Cheng C, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666-1675.
doi pubmed - Henderson LA, Degar BA. HLH treatment: smarter, not harder. Blood. 2022;139(24):3453-3455.
doi pubmed - Zhang Q, Zhao YZ, Ma HH, Wang D, Cui L, Li WJ, Wei A, et al. A study of ruxolitinib response-based stratified treatment for pediatric hemophagocytic lymphohistiocytosis. Blood. 2022;139(24):3493-3504.
doi pubmed - Boonstra PS, Ahmed A, Merrill SA, Wilcox RA. Ruxolitinib in adult patients with secondary hemophagocytic lymphohistiocytosis. Am J Hematol. 2021;96(4):E103-E105.
doi pubmed - Wang J, Zhang R, Wu X, Li F, Yang H, Liu L, Guo H, et al. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: a single-arm, multicentre, phase 2 trial. Br J Haematol. 2021;193(4):761-768.
doi pubmed - Cordas Dos Santos DM, Tix T, Shouval R, Gafter-Gvili A, Alberge JB, Cliff ERS, Theurich S, et al. A systematic review and meta-analysis of nonrelapse mortality after CAR T cell therapy. Nat Med. 2024;30(9):2667-2678.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.