| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 1, February 2025, pages 26-31
Successful Second Hematopoietic Stem Cell Transplantation Using Total Body Irradiation-Based Conditioning for Children With Transfusion-Dependent Beta-Thalassemia
Abdullah Al-Jefria, b, Khawar Siddiquia, Batool Al-Ghadeera, Amal Al-Seraihya, Ali Al-Ahmaria, Ibrahim Ghemlasa, Awatif AlAnazia, Hawazen Al-Saedia, Mahasen Saleha, Abdulrahman Al-Musaa, Mouhab Ayasa
aDepartment of Pediatric Hematology/Oncology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
bCorresponding Author: Abdullah Al-Jefri, Department of Pediatric Hematology/Oncology, King Faisal Specialist Hospital and Research Center, Riyadh 11211, Saudi Arabia
Manuscript submitted October 27, 2024, accepted January 15, 2025, published online January 25, 2025
Short title: Second HSCT in Pediatric Thalassemia
doi: https://doi.org/10.14740/jh1378
| Abstract | ▴Top |
Background: Graft rejection (GR) occurs in a significant proportion of individuals with transfusion-dependent β-thalassemia (TDT) following hematopoietic stem cell transplantation (HSCT). There have been limited data on the outcome and complications of second HSCT in β-thalassemia patients. The objective was to assess the survival benefits and outcome of second allogeneic HSCT in pediatric TDT patients using Cytoxan (CY) and total body irradiation (TBI) regimen.
Methods: This was a retrospective study on the analysis of the data for 15 patients who had graft failure over an 18-year period (March 2000 to March 2017) at our institution. For the first failed transplants for patients who had a myeloablative regimen consisting of busulfan (BU)-CY with or without additional anti-thymocyte globulin (ATG), the median age at transplant was 4.2 years. Graft failure occurred over a median of 8.6 months (range, 0.6 - 74.3 months) after the first transplant. The median time to the second transplant from GR was 25.3 months. For the second transplant, the same human leukocyte antigen (HLA) match-related donors for the first HSCT were used. Over half of the patients had moderate to severe iron overload with pre-transplant serum ferritin of 1,405 to 4,051 µg/L at transplant.
Results: Thirteen patients (86.7%) engrafted with thalassemia-free survival (TFS) of 80.0%. One patient rejected the graft and died. Another died due to infectious complications. Apart from a mild chronic graft-versus-host disease (GvHD) in one patient, no serious complications were observed.
Conclusion: CY-TBI can be used as conditioning for second HSCT in patients with TDT GR following myeloablative conditioning. We observed overall survival and TFS of 87% and 80% respectively with low rejection rate and mortality, and limited long-term side effects.
Keywords: Stem cell transplantation; Pediatric; Thalassemia; Second transplant; Graft failure; Survival benefits
| Introduction | ▴Top |
Hematopoietic stem cell transplantation (HSCT) is considered the potential curative treatment for patients with transfusion-dependent β-thalassemia (TDT) [1-3]. Advanced stage of disease, multiple transfusions over a long period of time, and age of the patient may adversely lead to graft rejection (GR) which can be as high as 38% following HSCT [4]. Recurrence of thalassemia with autologous marrow recovery usually follows GR; however, marrow aplasia may occur [4-8]. Limited publications on second HSCT in TDT are available [9]. Different conditioning regimens had been tried aiming to increase thalassemia-free survival (TFS) and overall survival (OS) and at the same time to decrease regimen-related toxicity and graft failure with variable results. However, the outcome was poor when using less intensive conditioning [4, 6, 9, 10]. We therefore reviewed our experience on second transplantation after graft failure using Cytoxan (CY) and total body irradiation (TBI) regimen as myelo-immunoablative regimen and compared it with the outcome of the published data.
| Materials and Methods | ▴Top |
From March 2000 to March 2017, 15 pediatric TDT patients (age ≤ 14 years) underwent second HSCT at our institution following the failure of their first transplant that was offered at the same center. We gathered clinical, transplant-related, toxicity and outcome data on these patients from the institutional databases being populated prospectively. Ten patients were female (66.7%); patient characteristics and transplant-related parameters for the first and second transplant are provided in Table 1. All recipients were conditioned with myeloablative regimen consisting of busulfan (BU) 16 mg/kg, CY 200 mg/kg and anti-thymocyte globulin (ATG, Grafalon) 40 mg/kg in the first transplant and for the second transplantation CY 200 mg/kg over 4 days and TBI of 1,200 cGy fractionated in six doses over 3 days with lung shielding.
 Click to view | Table 1. Patient Characteristics and Transplant-Related Parameters (n = 15) |
For the second transplant, bone marrow (BM) was the source of stem cells in 14 (93.3%) and one patient received peripheral blood stem cells (PBSCs). Donor was a human leukocyte antigen (HLA) identical sibling in 13 (86.7%) transplants and remaining two were parental donors; one was a mother and the other a father, in each. Donor-recipient degree of HLA mismatch was 10/10 in all cases. Median CD34+ cell dose was 4.6 × 106/kg (range, 2.4 - 20.5). Following the graft granulocyte colony-stimulating factor (G-CSF) was administered in 11 cases. The median interval between the first HSCT and graft failure was 8.6 months (0.6 - 74.3 months) and the same between graft failure and the second HSCT was 25.3 months (7.8 - 115.1 months). Median time to the second transplant from the first was 47.2 months (range, 21.3 - 125.3). Liver biopsies were not done routinely for the patients; therefore, Pesaro classification was not possible. However, severity was assessed based on risk category (ferritin > 3,000 µg/L, liver size > 3 cm below costal margin and age at transplant > 7.0 years); presence of three risk factors was considered severe, of two moderate and one mild. More than half of the patients were in the moderate to severe iron overload with serum ferritin range of 1,405 to 4,051 µg/L at transplant. None of our patients had splenectomy. All patients were on transfusion protocol in the interim between graft failure and the second transplant and were on iron chelation therapy in the form of oral deferasirox throughout interim period and were monitored routinely by serum ferritin every 3 months as follow-up assessment. Routinely, patients were given cytomegalovirus (CMV) prophylaxis in the form of intravenous (IV) acyclovir until day 100.
Statistical analysis
All continuous data are presented as median with minimum and maximum points. Kaplan-Meier curve was drawn for survival analysis and death from all causes was considered as an event. Graft failure or death from all causes was considered as event to estimate TFS. IBM-SPSS for Windows (version 20.0) was used for statistical analysis of the data.
Institutional Review Board approval and ethical compliance
This study was submitted to the Institutional Review Board of King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, and was approved by the Research Advisory Committee through established procedures via approval number 2211115. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Following the second transplantation, all our recipients had successful engraftment with neutrophil recovery in a median of 18 days (range, 13 -22), while platelets recovery was recorded in 11 (73.3%) with a median of 35 days (range, 19 - 96); seven (63.6%) of them achieved this within day +42. Based on hematopoietic assessment and short tandem repeat parameters of > 60% donor cells, 13 (86.7%) engrafted on day +100, one (6.7%) had a primary graft failure and the remaining one (6.7%) had a secondary graft failure. Cumulative incidence of acute graft-versus-host disease (GvHD) was 33.3% (n = 5) and only one recipient (6.7%) had limited mild chronic GvHD of skin. Details on transplant-related complications and infectious toxicity within day +100 are also provided in Table 1. At the last follow-up, 13 (86.7%) of the recipients were maintaining their graft with no evidence of disease or transfusion dependence. For two recipients with graft failure, one child with secondary graft failure died later and the other was alive and transfusion-dependent. Median follow-up time was 137.9 months (95% confidence interval: 126.0 - 150.0 months). With a mortality rate of 13.3% (n = 2), the cumulative probability of 5-year OS was 92.9±6.9%. The same for TFS was 80.0±10.3% (Fig. 1). Causes of death included disseminated fungal infection with CMV viremia in one and pulmonary aspergillosis and multi-organ failure in the other patient. Snapshot of the recipients and post-transplant outcome are described in Table 2. Post-transplant on follow-up growth hormones deficiency was noted in four (26.7%) and gonadal failure in five (33.3%) recipients only.
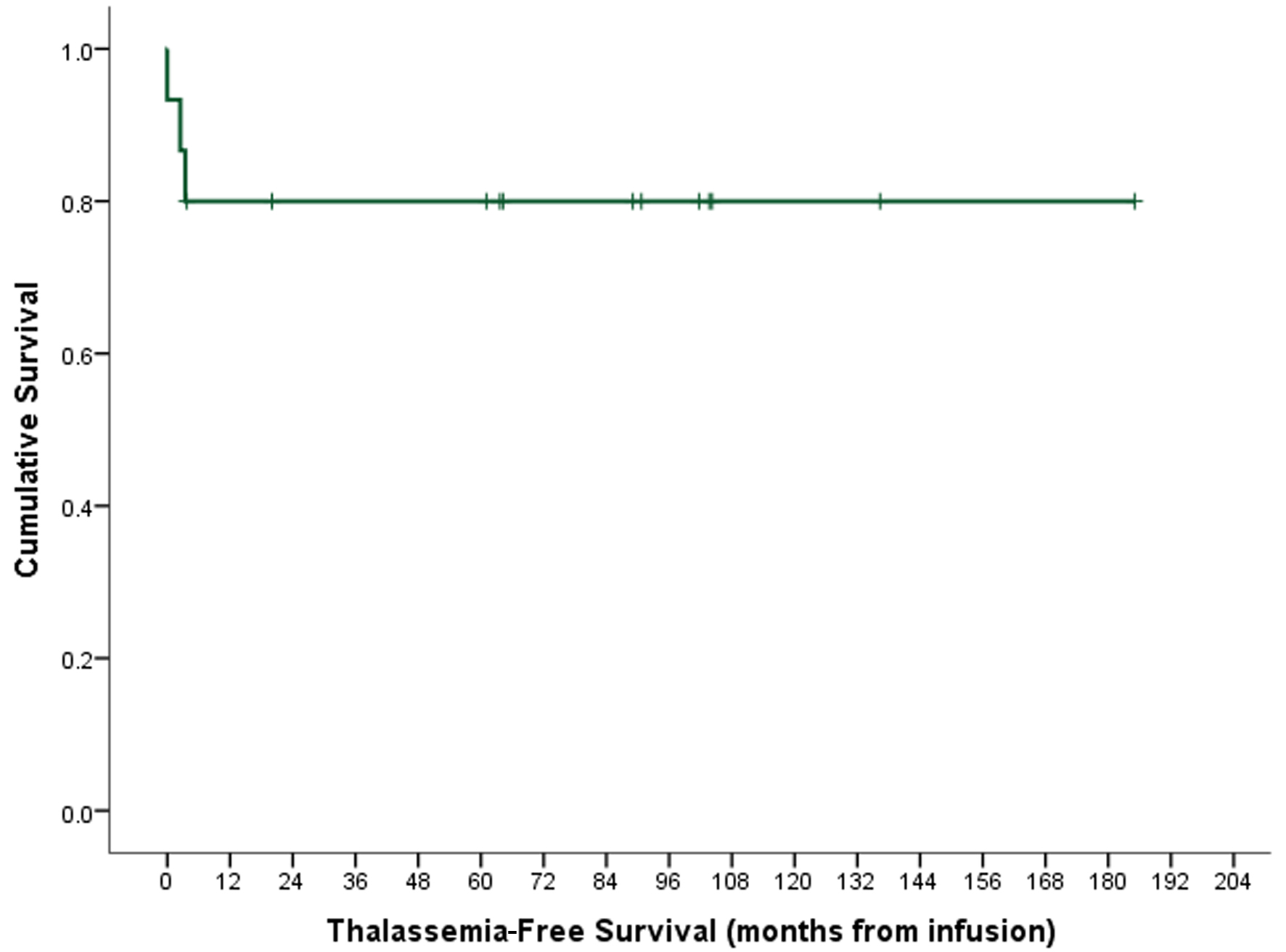 Click for large image | Figure 1. Thalassemia-free survival. |
 Click to view | Table 2. Snapshot of the Cohort for Second Transplant Episode (n = 15) |
| Discussion | ▴Top |
The use of a myeloablative conditioning for patients with TDT utilizing BU/CY had proven to be effective and successful regimen in this disease category. However, rejection remains a challenge beside the long-term toxicity [4, 6, 10, 11]. A study reported the outcome of the use of reduced-intensity conditioning (RIC) regimen to avoid toxicity, resulting in an increased rejection rate up to 63% [10]. Stepensky et al in 2009 reported unfavorable outcome using different RIC regimens on nine patients who had a second HSCT after rejection. Following HSCT, 33% had early mortality and 66% developed GvHD, while only 40% were transfusion-independent with stable chimerism [9]. Gaziev et al reported on 16 patients who had a second HSCT using intensive myelo-immunoablation regimen using BU/CY including azathioprine, hydroxyurea, ATG, thiotepa, fludarabine, pre-transplant and prolonged immunosuppression post-HSCT (protocol 26). The OS, event-free survival, treatment-related mortality and graft failure rates were 79%, 79%, 16%, and 6%, respectively. The study confirmed the importance of conditioning regimen intensification for a successful second engraftment [6]. Other studies had also indicated the need to use both myeloablation and immunosuppression regimens [12-14]. It is crucial to give adequate time interval between the two transplants to allow organ recovery, and to reduce toxicity and transplant-related mortality [9]. Another study had shown that the outcome is significantly better if the second HSCT is deferred for more than 1 year after graft failure [15]. In our study, median time to the second transplant from the first was 47.2 months (range, 21.3 - 125.3).
Having failed conditioning with BU/CY for the first HSCT in our cohort of patients, we elected to use combination of CY/TBI in an attempt to intensify the myeloablation/immunoablation effect. Inclusion of radiation to conditioning has been known to improve donor cell engraftment and decrease the risk of GR by its potent immunosuppressive effect. However, it can cause immediate and delayed toxicities and overlap with side effects of other chemotherapies used in conditioning regimen. Delayed complications such as pulmonary toxicity, infertility (gonadal dysfunction), growth and developmental delay, hyperthyroidism, and secondary malignancy can occur. However, the use of adjusted delivered dose, dose rate, fractionation, interval between fractions, lung and gonads shielding and the source of radiation all, can be practiced to reduce incidence of TBI-related side effects. A recent study using helical tomotherapy with gonadal sparing irradiation in conditioning for non-malignant disease demonstrated encouraging results in improved preservation of gonadal function [16].
Conclusion
To the best of our knowledge, this is the first long-term follow-up study using CY/TBI conditioning for second transplantation in patients following recurrence of thalassemia with good TFS and limited toxicity. Besides, TBI-based conditioning resulted in low rejection rate and mortality. All patients at their last follow-up were maintaining good quality of life and none of them developed any malignancy. Although this study is the longest follow-up evaluating outcome of a second transplantation following TBI-based regimen after rejection in TDT patients, further follow-ups with more patients are needed to validate our results.
Acknowledgments
None to declare.
Financial Disclosure
This work did not receive any financial support in any form from any funding agency.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Data of interest collected from the patients’ medical records were secured as governed by the institutional policies on patient confidentiality and privacy. No informed consents were obtained since this was a retrospective review of data and all data items collected were already documented in medical charts as part of the patients care and disease management documentation.
Author Contributions
All authors certify that they have participated sufficiently in the intellectual content and the analysis of data. Each author has reviewed the final version of the manuscript and approves it for publication. Should the editors request the data upon which the work is based, the authors shall produce it. AAJ and BAG conceived the idea, KS designed the study, prepared the database, analyzed the data and contributed towards results reporting and manuscript preparation and review. BAG collected the data and prepared the manuscript. AAJ supervised the study progression. AAS, AAA, IAG, AAA, HAS, MAS, AAM and MA reviewed the results and critically inspected the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ATG: anti-thymocyte globulin; BU: busulfan; CY: Cytoxan; GR: graft rejection; HSCT: hematopoietic stem cell transplantation; RIC: reduced-intensity conditioning; TBI: total body irradiation; TDT: transfusion-dependent β-thalassemia; TFS: thalassemia-free survival
| References | ▴Top |
- Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, Politi P, et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322(7):417-421.
doi pubmed - Martin A, Thompson AA. Thalassemias. Pediatr Clin North Am. 2013;60(6):1383-1391.
doi pubmed - Thomas ED, Buckner CD, Sanders JE, Papayannopoulou T, Borgna-Pignatti C, De Stefano P, Sullivan KM, et al. Marrow transplantation for thalassaemia. Lancet. 1982;2(8292):227-229.
doi pubmed - Korula A, Pn N, Devasia A, Lakshmi KM, Abraham A, Sindhuvi E, George B, et al. Second hematopoietic stem cell transplant for thalassemia major: improved clinical outcomes with a treosulfan-based conditioning regimen. Biol Blood Marrow Transplant. 2018;24(1):103-108.
doi pubmed - Andreani M, Testi M, Lucarelli G. Mixed chimerism in haemoglobinopathies: from risk of graft rejection to immune tolerance. Tissue Antigens. 2014;83(3):137-146.
doi pubmed - Gaziev J, Sodani P, Lucarelli G, Polchi P, Marktel S, Paciaroni K, Marziali M, et al. Second hematopoietic SCT in patients with thalassemia recurrence following rejection of the first graft. Bone Marrow Transplant. 2008;42(6):397-404.
doi pubmed - Horan JT, Liesveld JL, Fenton P, Blumberg N, Walters MC. Hematopoietic stem cell transplantation for multiply transfused patients with sickle cell disease and thalassemia after low-dose total body irradiation, fludarabine, and rabbit anti-thymocyte globulin. Bone Marrow Transplant. 2005;35(2):171-177.
doi pubmed - Sodani P, Gaziev D, Polchi P, Erer B, Giardini C, Angelucci E, Baronciani D, et al. New approach for bone marrow transplantation in patients with class 3 thalassemia aged younger than 17 years. Blood. 2004;104(4):1201-1203.
doi pubmed - Stepensky P, Or R, Shapira MY, Revel-Vilk S, Stein J, Resnick IB. Second bone marrow transplantation for patients with thalassemia: risks and benefits. Haematologica. 2009;94(9):1329-1330.
doi pubmed - Gaziev D, Polchi P, Lucarelli G, Galimberti M, Sodani P, Angelucci E, Giardini C, et al. Second marrow transplants for graft failure in patients with thalassemia. Bone Marrow Transplant. 1999;24(12):1299-1306.
doi pubmed - Andreani M, Testi M, Battarra M, Indigeno P, Guagnano A, Polchi P, Federici G, et al. Relationship between mixed chimerism and rejection after bone marrow transplantation in thalassaemia. Blood Transfus. 2008;6(3):143-149.
doi pubmed - Ramsay NK, Kim T, Nesbit ME, Krivit W, Coccia PF, Levitt SH, Woods WG, et al. Total lymphoid irradiation and cyclophosphamide as preparation for bone marrow transplantation in severe aplastic anemia. Blood. 1980;55(2):344-346.
pubmed - Storb R, Etzioni R, Anasetti C, Appelbaum FR, Buckner CD, Bensinger W, Bryant E, et al. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood. 1994;84(3):941-949.
pubmed - Iannone R, Casella JF, Fuchs EJ, Chen AR, Jones RJ, Woolfrey A, Amylon M, et al. Results of minimally toxic nonmyeloablative transplantation in patients with sickle cell anemia and beta-thalassemia. Biol Blood Marrow Transplant. 2003;9(8):519-528.
doi pubmed - Bolanos-Meade J, Cooke KR, Gamper CJ, Ali SA, Ambinder RF, Borrello IM, Fuchs EJ, et al. Effect of increased dose of total body irradiation on graft failure associated with HLA-haploidentical transplantation in patients with severe haemoglobinopathies: a prospective clinical trial. Lancet Haematol. 2019;6(4):e183-e193.
doi pubmed - Dibs K, Sim AJ, Penagaricano JA, Latifi K, Garcia GA, Peters JA, Nieder ML, et al. Gonadal-sparing total body irradiation with the use of helical tomotherapy for nonmalignant indications. Rep Pract Oncol Radiother. 2021;26(1):153-158.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.