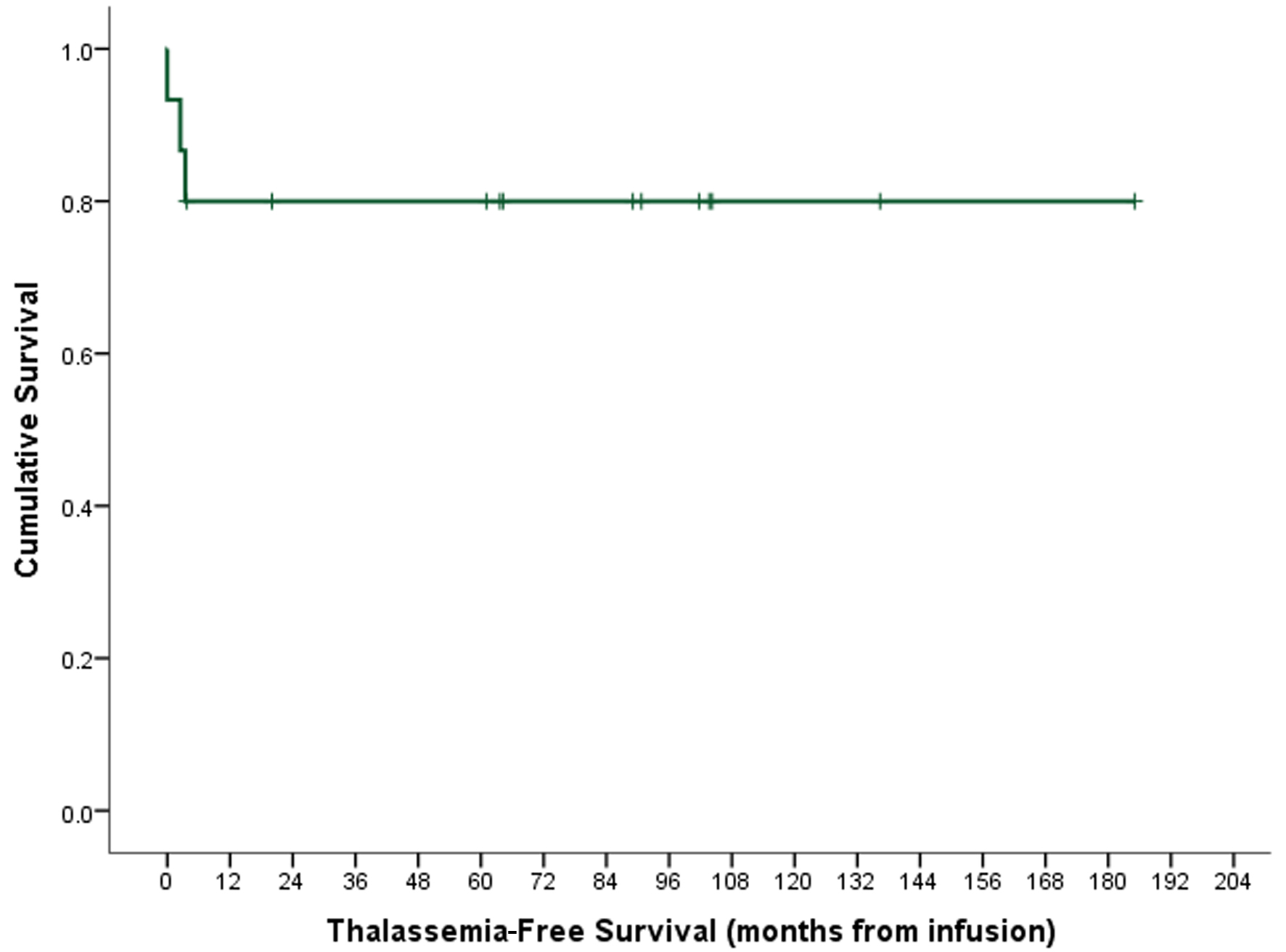
Figure 1. Thalassemia-free survival.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 1, February 2025, pages 26-31
Successful Second Hematopoietic Stem Cell Transplantation Using Total Body Irradiation-Based Conditioning for Children With Transfusion-Dependent Beta-Thalassemia
Figure

Tables
| Parameters of interest, n (%) | First transplant | Second transplant | P value |
|---|---|---|---|
| ANC: absolute neutrophil count; ATG: anti-thymocyte globulin; BU: busulfan; CMV: cytomegalovirus; CY: Cytoxan; GvHD: graft-versus-host disease; PBSC: peripheral blood stem cell; SCT: stem cell transplantation; TBI: total body irradiation. | |||
| Age at SCT, years, median (range) | 4.2 (2.0 - 9.9) | 10.1 (5.3 - 13.8) | - |
| Source of stem cells | 1.000 | ||
| Bone marrow | 15 (100.0%) | 14 (93.3%) | |
| PBSC | - | 1 (6.7%) | |
| CD34 (106/kg), median (range) | 8.5 (3.0 - 12.7) | 4.6 (2.4 - 20.5) | - |
| Recipient weight (kg) at infusion, median (range) | 15.5 (9.0 - 28.2) | 24.9 (15.1 - 48.9) | - |
| Conditioning regimens | < 0.001 | ||
| BU, CY | 8 (53.3%) | - | |
| BU, CY, ATG | 7 (46.7%) | - | |
| CY, TBI | - | 14 (93.3%) | |
| CY, TBI, ATG | - | 1 (6.7%) | |
| Donor → recipient gender | 1.000 | ||
| Male → female | 3 (20.0%) | 3 (20.0%) | |
| Male → male | 2 (13.3%) | 3 (20.0%) | |
| Female → female | 7 (46.7%) | 7 (46.7%) | |
| Female → male | 3 (20.0%) | 2 (13.3%) | |
| Time to ANC recovery, median days (range), number recovered | 16 (13 - 27), 15 | 18 (13 - 22), 15 | |
| Time to platelets recovery, median days (range), number recovered | 29 (15 - 54), 15 | 35 (19 - 96), 11 | 0.348 |
| Within 42 days | 13 (86.7%) | 7 (63.6%) | |
| Beyond 42 days | 2 (13.3%) | 4 (36.4%) | |
| Graft evaluation at day +100 | 0.080 | ||
| Engrafted | 9 (60.0%) | 13 (86.7%) | |
| Primary graft failure | - | 1 (6.7%) | |
| Secondary graft failure | 6 (40.0%) | 1 (6.7%) | |
| Acute GvHD (+) | 1 (6.7%) | 5 (33.3%) | 0.169 |
| Acute GvHD overall | 1.000 | ||
| Grade I | 1 (100.0%) | 2 (40.0%) | |
| Grade II | - | 1 (20.0%) | |
| Grade III | - | 2 (40.0%) | |
| Transplant-related toxicity during day +100 | |||
| Hypertension | 9 (60.0%) | 12 (80.0%) | 0.427 |
| Seizure | - | 2 (13.3%) | 0.483 |
| Interstitial pneumonia | 1 (6.7%) | - | 1.000 |
| Hemorrhagic cystitis | 2 (13.3%) | 1 (6.7%) | 1.000 |
| CMV reactivation | 3 (20.0%) | 3 (20.0%) | 1.000 |
| Encephalopathy | - | 1 (6.7%) | 1.000 |
| Mucositis | 1 (6.7%) | 9 (60.0%) | 0.005 |
| Infections | |||
| Bacterial | 4 (26.7%) | 3 (20.0%) | 1.000 |
| Viral | 1 (6.7%) | 3 (20.0%) | 0.598 |
| Fungal | 2 (13.3%) | 1 (6.7%) | 1.000 |
| Chronic GvHD | - | 1 (6.7%) | 1.000 |
| Graft evaluation at last contact | < 0.001 | ||
| Engrafted | - | 13 (86.7%) | |
| Failed graft | 15 (100.0%) | 2 (13.4%) | |
| Pt. | Sex | Age at transplant (years) | Months to second transplant | CD34 (106/kg) | Conditioning | Source | Donor | Growth factors | Days to ANC recovery | Days to PLT recovery | Day +100 engraftment | Ferritin (µg/L) | T2* cardiac (ms) | T2* hepatic (ms) | %Mye (D +365) | %Lym (D +365) | Growth hormones deficiency | %Mye latest | %Lym latest | OS (years from first infusion) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A: alive; ANC: absolute neutrophil count; ATG: anti-thymocyte globulin; BM: bone marrow; BU: busulfan; E: expired; G-CSF: granulocyte colony-stimulating factor; Lym: lymphoid; Mye: myeloid; OS: overall survival; PBSC: peripheral blood stem cell; PLT: platelets; PriGF: primary graft failure; SecGF: secondary graft failure; TBI: total body irradiation. | ||||||||||||||||||||
| 2 | M | 7.4 | 65.6 | 10.66 | CY/TBI | BM | Brother | G-CSF | 21 | 29 | Engrafted | 1,560 | 100 | 100 | -ve | 100 | 100 | A, 20.6 | ||
| 4 | F | 11.9 | 119.7 | 2.51 | CY/TBI | BM | Brother | G-CSF | 19 | - | PriGF | 1,222 | 30.27 | 5.28 | 0.0 | 0.0 | -ve | 0 | 0 | A, 16.6 |
| 6 | F | 10.6 | 96.4 | 2.56 | CY/TBI | BM | Sister | G-CSF | 16 | 36 | Engrafted | 684 | 100 | 100 | -ve | 100 | 100 | A, 13.2 | ||
| 8 | F | 5.7 | 26.8 | 3.95 | CY/TBI | BM | Sister | G-CSF | 19 | 52 | Engrafted | 1,405 | 99.4 | 98.9 | -ve | 99 | 99 | A, 2.5 | ||
| 10 | M | 9.7 | 82.1 | 4.57 | CY/TBI | BM | Sister | G-CSF | 13 | 35 | Engrafted | 470 | 100 | 100 | -ve | 100 | 100 | A, 11.8 | ||
| 12 | F | 10.1 | 47.2 | 4.24 | CY/TBI | BM | Sister | -ve | 20 | 96 | Engrafted | 1,057 | 100 | 100 | +ve | 13 | 41 | A, 11.3 | ||
| 14 | M | 5.3 | 27.1 | 7.62 | CY/TBI | BM | Brother | -ve | 22 | 30 | Engrafted | 3,833 | 12.0 | 58.0 | +ve | 100 | 100 | A, 10.7 | ||
| 16 | F | 7.7 | 42.4 | 20.48 | CY/ATG/TBI | PBSC | Mother | G-CSF | 13 | 19 | Engrafted | 4,051 | 30.2 | 2.72 | 100 | 100 | -ve | 100 | 100 | A, 5.1 |
| 18 | F | 9.1 | 55.0 | 4.57 | CY/TBI | BM | Sister | G-CSF | 18 | - | Engrafted | 1,356 | - | - | -ve | 100 | 100 | E, 4.7 | ||
| 20 | F | 12.0 | 25.6 | 2.37 | CY/TBI | BM | Sister | G-CSF | 15 | 24 | Engrafted | 1,269 | 100 | 100 | +ve | 100 | 100 | A, 9.4 | ||
| 22 | M | 11.9 | 32.0 | 2.77 | CY/TBI | BM | Brother | -ve | 21 | 44 | Engrafted | 2,924 | 100 | 100 | -ve | 100 | 93 | A, 11.2 | ||
| 24 | F | 13.8 | 125.3 | 4.94 | CY/TBI | BM | Brother | G-CSF | 14 | - | Engrafted | 2,793 | 36.45 | 1.5 | 100 | 100 | -ve | 100 | 100 | A, 15.5 |
| 26 | F | 13.8 | 111.3 | 4.79 | CY/TBI | BM | Father | -ve | 13 | - | SecGF | 1,020 | 36.0 | 8.8 | - | - | -ve | 100 | 61 | E, 9.6 |
| 29 | F | 9.9 | 39.2 | 3.96 | CY/TBI | BM | Sister | G-CSF | 19 | 29 | Engrafted | 2,028 | 100 | 100 | -ve | 100 | 100 | A, 14.4 | ||
| 31 | M | 10.8 | 21.3 | 8.8 | CY/TBI | BM | Sister | G-CSF | 16 | 58 | Engrafted | 1,504 | 100 | 100 | +ve | 100 | 100 | A, 10.1 | ||