| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 14, Number 1, February 2025, pages 20-25
Increased Transferrin Concentrations Are Not Associated With Thrombosis in People Living at High Altitude
Ricardo Amarua, b, g, Josef Prchalc, Tomas Ganzd, Xu Zhange, Daniela Patona, Mireya Carrascob, Emma Mancillac, Victor R. Gordeuke, g
aCell Biology Unit, School of Medicine, San Andres University, La Paz, Bolivia
bInstituto Boliviano de Oncohematologia, La Paz City, Bolivia
cDepartment of Medicine, University of Utah, VAH, and Huntsman Cancer Institute, Salt Lake City, UT, USA
dUCLA Center for Iron Disorders, UCLA Department of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, David Geffen School of Medicine, Los Angeles, CA, USA
eDepartment of Medicine, University of Illinois at Chicago, IL, USA
fStatistics Department, School of Mathematical and Natural Sciences, San Andres University, La Paz, Bolivia
gCorresponding Author: Ricardo Amaru, Cell Biology Unit, School of Medicine, San Andres University, La Paz, Bolivia; Victor R. Gordeuk, Department of Medicine, University of Illinois at Chicago, IL, USA
Manuscript submitted November 15, 2024, accepted December 28, 2024, published online January 17, 2025
Short title: Transferrin and Thrombosis at High Altitude
doi: https://doi.org/10.14740/jh1388
| Abstract | ▴Top |
Background: Bolivian Andean Aymara highlanders, living at 4,000 m for 14,000 years, have evolved genetic adaptations to hypoxia. These include EGLN1 encoding prolyl hydroxylase 2 (PHD2), a regulator of transferrin transcription. Transferrin level increases in hypoxia and iron deficiency. Contrasting reports indicate that elevated transferrin is associated with experimentally induced thrombosis in mice undergoing short-stay at high altitude, but with decreased thrombosis in a congenital disorder of hypoxia-sensing.
Methods: A retrospective study was conducted in people living at high altitude (3,650 - 4,150 m). We analyzed serum transferrin concentration and thrombosis history in Aymara patients with high-altitude erythrocytosis (n = 149, median age 55 years, female gender 30%, iron deficiency 23%) or high-altitude anemia (n = 137, median age 43 years, female gender 86%, iron deficiency 57%).
Results: The median transferrin concentration was 339 mg/dL in erythrocytosis patients versus 310 mg/dL in anemia patients (P = 0.037); it was 367 mg/dL in iron deficient versus 312 mg/dL in iron replete patients (P < 0.001). Thrombosis history was present in 13% of erythrocytosis and 8% of anemia patients (P = 0.25) and was present in 16% of iron deficient and 7% of iron replete patients (P = 0.017). After adjustment for erythrocytosis and iron deficiency in multivariate regression analysis, the mean (95% confidence interval) transferrin concentration was 277 (237 - 316) mg/dL in 30 patients with thrombosis history versus 324 (306 - 341) mg/dL in 256 patients without thrombosis history (P = 0.018). Similar trends occurred for the subgroups of arterial thrombosis history (P = 0.044) and venous thrombosis history (P = 0.22).
Conclusions: In individuals with extreme environmental hypoxia, we found no evidence that increased transferrin is associated with increased thrombosis history. Rather, we observed a trend to decreased thrombosis history with increased transferrin levels.
Keywords: Transferrin; Ferritin; Iron deficiency; Thrombosis; Hypoxia; High altitude
| Introduction | ▴Top |
Native Bolivian Aymaras have lived at approximately 4,000 m in the Andes Mountains for about 14,000 years, exposed to low atmospheric pressure and a corresponding decrease in atmospheric partial pressure of oxygen, i.e., hypobaric hypoxia. Evolutionary genetic adaptation to high altitude in Aymara Andeans involves cardiovascular system genes [1], with less prominent selection of hypoxia-inducible factor (HIF) pathway genes including EPAS1 encoding HIF-2α and EGLN1 encoding prolyl hydroxylase 2 (PHD2) [2]. HIF1A is also upregulated at high altitude [3, 4] and correlates with an Aymara evolutionary-selected NFKB1 haplotype encoding loss of function alternate NFKB1 transcripts that augment both HIFs and inflammation [5].
HIFs activate the transcription of genes that increase oxygen delivery and facilitate metabolic adaptation to hypoxia. There are three HIFs, HIF-1, HIF-2, and HIF-3, with HIF-1 and HIF-2 the best studied. HIFs are composed of α and β subunits. PHD2 is an iron-, α-ketoglutarate-, and oxygen-dependent enzyme that decreases HIFs by hydroxylating HIF-α subunits, a step that enables HIF recognition by von Hippel-Lindau (VHL) protein and its subsequent proteasomal degradation [6].
Transferrin, which is responsible for iron transport in the blood and iron delivery to cells through transferrin receptor-mediated endocytosis, is induced by both hypoxia and iron deficiency via HIF-1 [7]. Transferrin levels are increased in people who live at high altitude [8]. Iron deficiency itself is a risk factor for thrombosis as recently reviewed [9].
Native Andeans are primarily composed of two large groups, Aymaras predominating in Bolivia and Argentina and Quechuas predominating in Peru, Chile, and Ecuador. Both groups have adapted by increased hemoglobin concentrations compared to sea-level inhabitants. Normal hemoglobin levels in healthy highlanders range from 15 - 18 g/dL in males and 14 - 17 g/dL in females, which are 3 - 4 g/dL higher than sea-level values [10, 11]. Andeans may be predisposed to thromboembolic complications as environmental hypoxia is an independent risk factor for postoperative deep vein thrombosis and pulmonary embolism in persons living at high altitude [12], and for venous and arterial thrombosis in lowlander persons who move from low to high altitude [13]. While Quechuas and Aymaras share some evolutionary genetic adaptations, others differ between the Quechuas [14] and the Aymaras [1]. To date, no evidence has been published of increased thromboembolism in Bolivian Aymaras living at 4,000 m versus those living at sea level [12].
It has recently been proposed that the plasma iron transporter, transferrin, is a prothrombotic protein that promotes blood coagulation when abnormally increased [15, 16]. Specifically, it was reported that transferrin induces hypercoagulability by potentiating thrombin/factor XIIa interaction and by inhibiting antithrombin [15]. Furthermore, it was proposed that this mechanism contributes to thromboembolism at high altitude [8], and that pharmacological blockade or neutralization of transferrin may reverse the increased coagulation tendency observed under hypoxic conditions. Mice kept in hypoxic conditions had increased thrombosis in carotid arteries and deep veins, which could be reversed with anti-transferrin antibodies and peptides that block transferrin binding to thrombin and FXIIa [8]. However, a prospective study of 155 people with Chuvash erythrocytosis, a condition with genetically augmented hypoxic responses at normal altitude and ambient O2 [17], reported that elevated transferrin was associated with reduced thrombosis. Chuvash erythrocytosis is the first described inherited condition with augmented HIF levels [18], and thromboses are the principal cause of morbidity and mortality [19]. Other studies have also provided evidence for a protective role of transferrin against thrombosis [20, 21].
In the present report, we examine the relationship of increased circulating transferrin concentrations with a history of clinically documented thrombosis under the extreme conditions of high altitude with or without concomitant iron deficiency.
| Materials and Methods | ▴Top |
This retrospective study was conducted in people living at high altitude in two locations in Bolivia from January 2018 to September 2023: La Paz (11,942 ft, 3,650 m) and its suburb El Alto (13,615 ft, 4,150 m). We analyzed the relationship of serum transferrin concentration with history of thrombosis in Aymaras born and residing at these two high altitudes, who were found to have anemia (n = 137) or increased hemoglobin (n = 149). Anemia was defined as hemoglobin < 13 g/dL in women and < 14 g/dL in men (high-altitude anemia), and erythrocytosis was defined as hemoglobin levels > 18 g/dL in women and > 19 g/dL in men (high-altitude erythrocytosis) [10]. Iron deficiency was defined as serum ferritin < 30 µg/L and mean corpuscular volume (MCV) < 80 fL (thalassemia is not present in Andean South American regions) [22]. Echo-Doppler study records validated thrombotic events. Thromboses were categorized as venous (deep vein thrombosis, pulmonary thromboembolism and retinal) or arterial (ischemic stroke, acute myocardial infarction and mesenteric). This study was approved by the Institutional Ethics Committee of San Andres University School of Medicine and adhered to the principles set forth in the Declaration of Helsinki.
Data analysis was performed through Excel 16.29.1, SPSS, and SYSTAT programs. The association of serum transferrin with a history of thrombosis was tested by multivariate linear regression modeling, where covariates were determined by forward-backward model selection on variables which are individually correlated with serum transferrin or with a history of thrombosis at P = 0.1.
Clinical evaluations, laboratory tests, and echo-Doppler studies were performed in the routine care of patients not with the goal of testing a research hypothesis.
| Results | ▴Top |
There were 19 men and 118 women with high-altitude anemia, and 105 men and 44 women with high-altitude erythrocytosis, all with serum transferrin concentration measurements (Supplementary Material 1, jh.elmerpub.com). The 137 patients with high-altitude anemia were younger than the 149 patients with high-altitude erythrocytosis (median (interquartile range (IQR)) age of 43 (33 - 55) years versus 55 (49 - 64) years, P < 0.001), and they were more often females, 86% in the anemia cohort versus 30% in the erythrocytosis cohort (P < 0.001). Iron deficiency (defined by serum ferritin < 30 µg/L and MCV < 80 fL) was present in 57% of the patients with anemia versus 23% of those with erythrocytosis (P < 0001). Transferrin levels were slightly lower in anemia patients (median (IQR): 310 (210 - 401) mg/dL versus 339 (294 - 371) mg/dL in erythrocytosis patients, P = 0.037). A history of thrombosis, mainly venous, was present in 8.0% of the anemia patients compared to 13.0% of the erythrocytosis patients (P = 0.25). The history of venous thrombosis was found in 5.1% of the high-altitude anemia patients and 8.7% of the high-altitude erythrocytosis patients (P = 0.25). The history of arterial thrombosis was found in 3.7% of the high-altitude anemia patients and 4.0% of the high-altitude erythrocytosis patients (P = 1.0). One patient had a history of both venous and arterial thrombosis.
Transferrin levels were higher in the iron deficiency patients (median (IQR): 367 (278 - 426) mg/dL versus 312 (253 - 360) mg/dL in the iron replete patients, P < 0.001). A history of thrombosis was present in 16% of the iron deficiency patients compared to 6.9% of the iron replete patients (P = 0.017). The history of venous thrombosis was found in 9.8% of iron deficiency patients and 5.2% of iron replete patients (P = 0.16). The history of arterial thrombosis was found in 6.3% of the iron deficiency patients and 2.3% of the iron replete patients (P = 0.12) (Supplementary Material 2, jh.elmerpub.com).
We assessed the correlations of baseline characteristics with history of thrombosis, as shown in Table 1. Older age, lower lymphocyte count, iron deficiency, and lower serum ferritin were significantly associated with the history of thrombosis. We also assessed the correlation of these baseline characteristics with transferrin concentrations. As shown in Table 2, only high-altitude erythrocytosis versus anemia, low MCV, iron deficiency, and low serum ferritin, had significant associations with higher serum transferrin concentration.
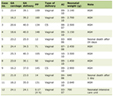 Click to view | Table 1. Demographics, Laboratory Studies, and Iron Status in 286 High Altitude Patients, 137 With Anemia and 149 With Erythrocytosis, According to the History of Thrombosis |
 Click to view | Table 2. Independent Correlations With Serum Transferrin Concentration in 286 High Altitude Patients, 137 With Anemia and 149 With Erythrocytosis |
We then assessed the relationship of serum transferrin concentration with history of thrombosis, with adjustment for erythrocytosis versus anemia and iron deficiency determined by model selection (Methods). As shown in Table 3, transferrin was higher in patients without a history of thrombosis (adjusted mean (95% confidence interval (CI)): 324 (306 - 341) mg/dL, compared to 277 (237 - 316) mg/dL in those with such a history and β (95% CI): 47 (8.3 - 86) mg/dL (P = 0.018)). There was also a trend of higher transferrin in the subgroups of patients without a history of venous thrombosis (P = 0.22) or arterial thrombosis (P = 0.044).
 Click to view | Table 3. Transferrin Concentrations (mg/dL; Median and IQR) According to the History of Thrombosis at High Altitude (Adjusted for Erythrocytosis Versus Anemia and Iron Deficiency) |
| Discussion | ▴Top |
Persons who move from low to high altitude have an increased risk of thrombosis [13], but it is not known if there is a difference in thrombosis between evolutionarily adapted high-altitude natives, Bolivian Aymaras, born and living at high altitude their entire life, compared to those living at sea level. We examined the relationships of iron deficiency and transferrin with history of thrombosis in Bolivian Aymara highlanders with either anemia or erythrocytosis.
Our findings confirm that iron deficiency is associated with increased serum concentrations of transferrin and with increased history of thrombosis in people living at high altitude. However, our data indicate that increased transferrin level is not part of the mechanism of increased thrombotic risk, and that it may even protect people from thrombosis.
Other reports provide support for our findings including Chuvash erythrocytosis, a congenital disorder causing upregulated HIFs and high thrombotic risk, wherein increased transferrin is associated with protection from thrombosis [17]. Furthermore, in Chuvash erythrocytosis, an intronic single nucleotide polymorphism (SNP) in TF, the gene for transferrin, is associated with higher transferrin levels and further protection from thrombosis [17], which strengthens the potential causal relationship between increased transferrin and a lower risk for thrombosis. In a study from Finland of over 2,000 subjects, higher total iron binding capacity (TIBC), which represents serum transferrin, was associated with decreased risk for acute myocardial infarction, while lower ferritin showed a trend for an increased risk [21]. In a meta-analysis of almost 50,000 subjects, higher serum transferrin was associated with a lower risk of venous thrombosis, but in contrast to our findings, higher serum ferritin was associated with thrombosis [20].
Transferrin elevation is induced by both iron deficiency and hypoxia via HIF signaling [7]. Iron deficiency enhances hypoxic responses by inhibiting the activity of iron- and oxygen-dependent prolyl hydroxylases (PHDs) that hydroxylate HIF-α subunits, permitting their recognition by VHL [6] and subsequent proteasomal degradation. Potential mechanisms for increased thrombosis in iron deficiency that do not involve elevated transferrin include elevated factor VIII [23], increased tissue factor [24], decreased KLF2 [25], chronically increased erythropoietin [17, 26], and thrombocytosis [27]. However, we did not observe a correlation between increased platelet count and history of thrombosis in any of the analyses in this study.
The discrepancy between studies describing transferrin as a risk factor for thrombosis in mice [8, 15, 16] and those describing transferrin as a protection from thrombosis in humans [17, 20, 21] may in part be explained by the fact that FeCl3-mediated induction of thrombosis in mice does not fully reflect thrombogenesis in humans [28].
One of the genes activated by HIF-1 and HIF-2 at high altitude is plasminogen activator inhibitor-1 (PAI-1), a regulator of numerous pathophysiological processes including thrombosis. It has been proposed that by inducing the expression of PAI-1, HIF-2 can contribute to thrombosis [29]. Similarly, in an acquired erythrocytosis, polycythemia vera, and in a related disorder, essential thrombocythemia, HIF activity is augmented in neutrophils and platelets and is associated with HIF-1-mediated upregulation of tissue factor and other prothrombotic genes [24, 30]. In these disorders transcription factor KLF2 that inhibits thrombosis is decreased, providing an additional mechanism of a prothrombotic milieu by augmented hypoxia signaling [25]. Here, we report the increased history of thrombosis in environmental hypobaric hypoxia.
In summary, our study of a population of Bolivian Aymaras exposed to environmental hypoxia confirms the association of iron deficiency with history of thrombosis, while raising questions about whether the elegant studies of prothrombotic effects of increased transferrin in mice are applicable to human subjects. In fact, like Chuvash erythrocytosis [17], increased transferrin may protect from thrombosis at high altitude in the Aymara population. Whether these findings can be generalized to non-highlanders or to populations without similar genetic adaptations to hypoxia will require further investigation.
There are several limitations to our study. The incidence of thrombosis was assessed retrospectively by means of chart review. The sample size for the subgroup of patients with a history of arterial thrombosis is relatively small, reducing statistical power and reliability of the subgroup analyses. We did not measure plasma levels of proteins involved in thrombotic events such as fibrinogen, PAI-1, KLF2 or tissue factor, and this limits the mechanistic insights that we can draw from our findings.
| Supplementary Material | ▴Top |
Suppl 1. Demographics, laboratory studies, and iron status in high altitude anemia and high altitude erythrocytosis patients.
Suppl 2. Demographics and laboratory variables according to iron deficiency in high altitude anemia and high altitude erythrocytosis patients.
Acknowledgments
The authors thank all of the physicians and data managers who were involved and contributed to the study.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
RA conceived the project, designed the study, supervised and interpreted data, wrote the manuscript, and finalized the submitted manuscript. EM analyzed and interpreted the data. JP helped to interpret the data, provided additional insights, and edited and approved the final manuscript. TG provided critical conceptual and statistical input and edited the manuscript. XZ provided statistical analysis and interpretation and edited the manuscript. DP wrote and edited the manuscript. MC collected the data, helped to interpret the data. VRG helped to write the manuscript, analyzed and interpreted the data, provided additional insights, and edited and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon request.
| References | ▴Top |
- Crawford JE, Amaru R, Song J, Julian CG, Racimo F, Cheng JY, Guo X, et al. Natural selection on genes related to cardiovascular health in high-altitude adapted andeans. Am J Hum Genet. 2017;101(5):752-767.
doi pubmed - Amaru R, Song J, Reading NS, Gordeuk VR, Prchal JT. "What we know and what we do not know about evolutionary genetic adaptation to high altitude hypoxia in andean aymaras". Genes (Basel). 2023;14(3):640.
doi pubmed - Soree P, Gupta RK, Singh K, Desiraju K, Agrawal A, Vats P, Bharadwaj A, et al. Raised HIF1alpha during normoxia in high altitude pulmonary edema susceptible non-mountaineers. Sci Rep. 2016;6:26468.
doi pubmed - Lu RL, Li P, Li B, Xing Y, Zhang YY, Chen BZ, Hu QN, et al. [Effects of altitude on circulating endothelial progenitor cells and hypoxia-inducible factor-1alpha in patients with type 2 diabetes]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2021;37(5):529-533.
doi pubmed - Song J, Han S, Amaru R, Lankova L, Quispe T, Kim D, et al. Elevated hemoglobin of andean aymaras is caused by alternatively spliced nfkb1. EHA Library.
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399-408.
doi pubmed - Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272(32):20055-20062.
doi pubmed - Li M, Tang X, Liao Z, Shen C, Cheng R, Fang M, Wang G, et al. Hypoxia and low temperature upregulate transferrin to induce hypercoagulability at high altitude. Blood. 2022;140(19):2063-2075.
doi pubmed - Kalff H, Cario H, Holzhauer S. Iron deficiency anemia and thrombosis risk in children-revisiting an old hypothesis. Front Pediatr. 2022;10:926925.
doi pubmed - Amaru R, Quispe T, Torres G, Mamani J, Aguilar M, Miguez H, et al. Caracterizacion clinica de la eritrocitosis patologica de altura. Revista de Hematologia. 2016;17(1):8-20.
- Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, et al. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol. 1998;106(3):385-400.
doi pubmed - Damodar D, Vakharia R, Vakharia A, Sheu J, Donnally CJ, 3rd, Levy JC, Kaplan L, et al. A higher altitude is an independent risk factor for venous thromboembolisms following total shoulder arthroplasty. J Orthop. 2018;15(4):1017-1021.
doi pubmed - Wojta J. High altitude thrombosis-Evidence for underlying mechanisms from a large prospective longitudinal study. Lancet Reg Health Southeast Asia. 2022;3:100039.
doi pubmed - Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, Zhao HW, et al. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet. 2013;93(3):452-462.
doi pubmed - Tang X, Zhang Z, Fang M, Han Y, Wang G, Wang S, Xue M, et al. Transferrin plays a central role in coagulation balance by interacting with clotting factors. Cell Res. 2020;30(2):119-132.
doi pubmed - Tang X, Fang M, Cheng R, Zhang Z, Wang Y, Shen C, Han Y, et al. Iron-deficiency and estrogen are associated with ischemic stroke by up-regulating transferrin to induce hypercoagulability. Circ Res. 2020;127(5):651-663.
doi pubmed - Shah BN, Zhang X, Sergueeva AI, Miasnikova GY, Ganz T, Prchal JT, Gordeuk VR. Increased transferrin protects from thrombosis in Chuvash erythrocytosis. Am J Hematol. 2023;98(10):1532-1539.
doi pubmed - Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, et al. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32(4):614-621.
doi pubmed - Gordeuk VR, Miasnikova GY, Sergueeva AI, Lorenzo FR, Zhang X, Song J, Stockton DW, et al. Thrombotic risk in congenital erythrocytosis due to up-regulated hypoxia sensing is not associated with elevated hematocrit. Haematologica. 2020;105(3):e87-e90.
doi pubmed - Gill D, Brewer CF, Monori G, Tregouet DA, Franceschini N, Giambartolomei C, Consortium I, et al. Effects of genetically determined iron status on risk of venous thromboembolism and carotid atherosclerotic disease: a mendelian randomization study. J Am Heart Assoc. 2019;8(15):e012994.
doi pubmed - Magnusson MK, Sigfusson N, Sigvaldason H, Johannesson GM, Magnusson S, Thorgeirsson G. Low iron-binding capacity as a risk factor for myocardial infarction. Circulation. 1994;89(1):102-108.
doi pubmed - Tang H, Zhang N, Liu X, Xiao H, Zhang H, Zhou K, Deng J. Incidence trends of inherited anemias at the global, regional, and national levels over three decades. J Epidemiol Glob Health. 2024;14(1):72-85.
doi pubmed - Livesey JA, Manning RA, Meek JH, Jackson JE, Kulinskaya E, Laffan MA, Shovlin CL. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax. 2012;67(4):328-333.
doi pubmed - Reeves BN, Kim SJ, Song J, Wilson KJ, Henderson MW, Key NS, et al. Tissue factor activity is increased in neutrophils from JAK2 V617F-mutated essential thrombocythemia and polycythemia vera patients. Am J Hematol. 2022;97(2):E37-E40.
- Song J, Kim SJ, Gollamudi J, Thiagarajan P, Prchal JT. Downregulated KLF2 in polycythemia vera and essential thrombocythemia induces prothrombotic gene expression. Blood Adv. 2023;7(5):712-717.
doi pubmed - Stockhausen S, Kilani B, Schubert I, Steinsiek AL, Chandraratne S, Wendler F, Eivers L, et al. Differential effects of erythropoietin administration and overexpression on venous thrombosis in mice. Thromb Haemost. 2024;124(11):1027-1039.
doi pubmed - Song AB, Kuter DJ, Al-Samkari H. Characterization of the rate, predictors, and thrombotic complications of thrombocytosis in iron deficiency anemia. Am J Hematol. 2020;95(10):1180-1186.
doi pubmed - Ciciliano JC, Sakurai Y, Myers DR, Fay ME, Hechler B, Meeks S, Li R, et al. Resolving the multifaceted mechanisms of the ferric chloride thrombosis model using an interdisciplinary microfluidic approach. Blood. 2015;126(6):817-824.
doi pubmed - Ahn YT, Chua MS, Whitlock JP, Jr., Shin YC, Song WH, Kim Y, Eom CY, et al. Rodent-specific hypoxia response elements enhance PAI-1 expression through HIF-1 or HIF-2 in mouse hepatoma cells. Int J Oncol. 2010;37(6):1627-1638.
doi pubmed - Gangaraju R, Song J, Kim SJ, Tashi T, Reeves BN, Sundar KM, Thiagarajan P, et al. Thrombotic, inflammatory, and HIF-regulated genes and thrombosis risk in polycythemia vera and essential thrombocythemia. Blood Adv. 2020;4(6):1115-1130.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.