| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 14, Number 1, February 2025, pages 32-37
A Rare Case of Acute Aleukemic Mast Cell Leukemia With Osteoblastic Lesions in the Appendicular Skeleton
Muralidhar Idamakantia, e , Ala Ebaidb, Rani Indrani Bijjama, Alexei Bakhirevc, Shiva Kumar Mukkamallad, Leslie Andritsosb
aAdult Internal Medicine Services (AIMS), Presbyterian Healthcare Services (PHS), Albuquerque, NM 87106, USA
bDivision of Hematology/Oncology, The University of New Mexico Comprehensive Cancer Center (UNMCC), Albuquerque, NM 87102, USA
cPathology Associates of Albuquerque (PAA), Albuquerque, NM 87106, USA
dHematology/Medical Oncology, UPMC Hillman Cancer Center, Indiana, PA 15701, USA
eCorresponding Author: Muralidhar Idamakanti, Adult Internal Medicine Services (AIMS), Presbyterian Healthcare Services (PHS), Albuquerque, NM 87106, USA
Manuscript submitted November 27, 2024, accepted January 2, 2025, published online January 17, 2025
Short title: Acute Aleukemic MCL With Osteoblastic Lesions
doi: https://doi.org/10.14740/jh1383
| Abstract | ▴Top |
Mast cell leukemia (MCL) is a rare and aggressive form of systemic mastocytosis (SM) that commonly involves the bone. This often presents as osteoporosis with focal osteolytic lesions and pathological fractures. Osteoblastic (sclerotic) lesions are rarely seen in MCL. The vertebral bodies are the most common site of bone involvement, with lesions outside of the axial skeleton being extremely rare. MCL presenting with osteoblastic lesions has been reported in the literature, however, there are no reported cases of osteoblastic lesions in the appendicular skeleton. Here we report a rare case of acute aleukemic MCL that presented with diffuse osteoblastic/sclerotic osseous lesions involving ribs, thoracic spine, lumbar spine and pelvis without pathological fractures.
Keywords: Mast cell leukemia; Systemic mastocytosis; Aleukemic mast cell leukemia; Osteoblastic osseous lesions; Tyrosine kinase inhibitors; Overall survival
| Introduction | ▴Top |
Mast cell leukemia (MCL) is a rare and aggressive form of systemic mastocytosis (SM) [1, 2]. Patients typically present with weight loss, fatigue, pain, flushing and gastrointestinal symptoms. Initial evaluation usually shows cytopenias, coagulopathy, hepatosplenomegaly, and skeletal involvement with osteoporosis, osteolytic lesions and/or pathological fractures [2-4]. Here we report a rare case of aleukemic MCL presented with diffuse osteoblastic/sclerotic osseous lesions involving ribs, thoracic spine, lumbar spine and pelvis without pathological fractures. We also briefly reviewed the diagnosis and treatment of MCL.
| Case Report | ▴Top |
This is a 75-year-old female with past medical history of obesity with body mass index (BMI) of 34.6 kg/m2, type 2 diabetes, essential hypertension, restless leg syndrome, hypothyroidism, fibromyalgia, chronic pain, and neuropathy, who presented to the emergency room with hypokalemia secondary to nausea, vomiting and diarrhea for 2 weeks. Review of systems is positive for mechanical fall the night prior to presentation secondary to bilateral leg weakness, diffuse pain in her neck, lower back, hip and epigastric abdominal area, and nausea and vomiting. Vital signs were stable, and basic labs showed pancytopenia, hypokalemia, mild hypocalcemia, and hyperbilirubinemia (Table 1). Computed tomography (CT) chest with contrast showed diffuse osteoblastic lesions in the ribs and vertebrae (Fig. 1a, b), and CT abdomen and pelvis with contrast revealed diffuse osteoblastic lesions in the pelvic bones (Fig. 2), hepatosplenomegaly and portal vein thrombosis (Fig. 3).
 Click to view | Table 1. Pertinent Laboratory Results on Presentation |
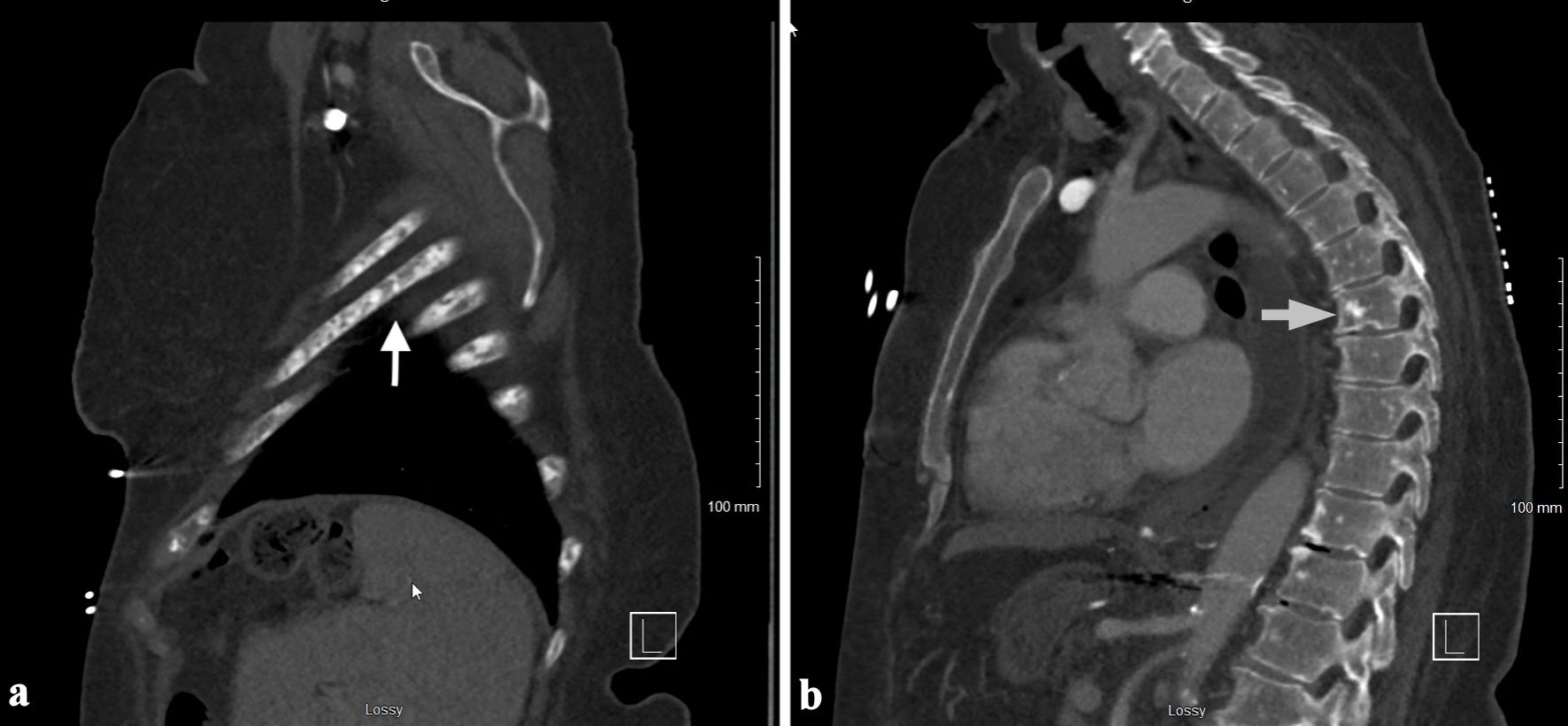 Click for large image | Figure 1. CT chest with contrast showing the osteoblastic lesions in the ribs (a) and vertebrae (b) (arrows). CT: computed tomography. |
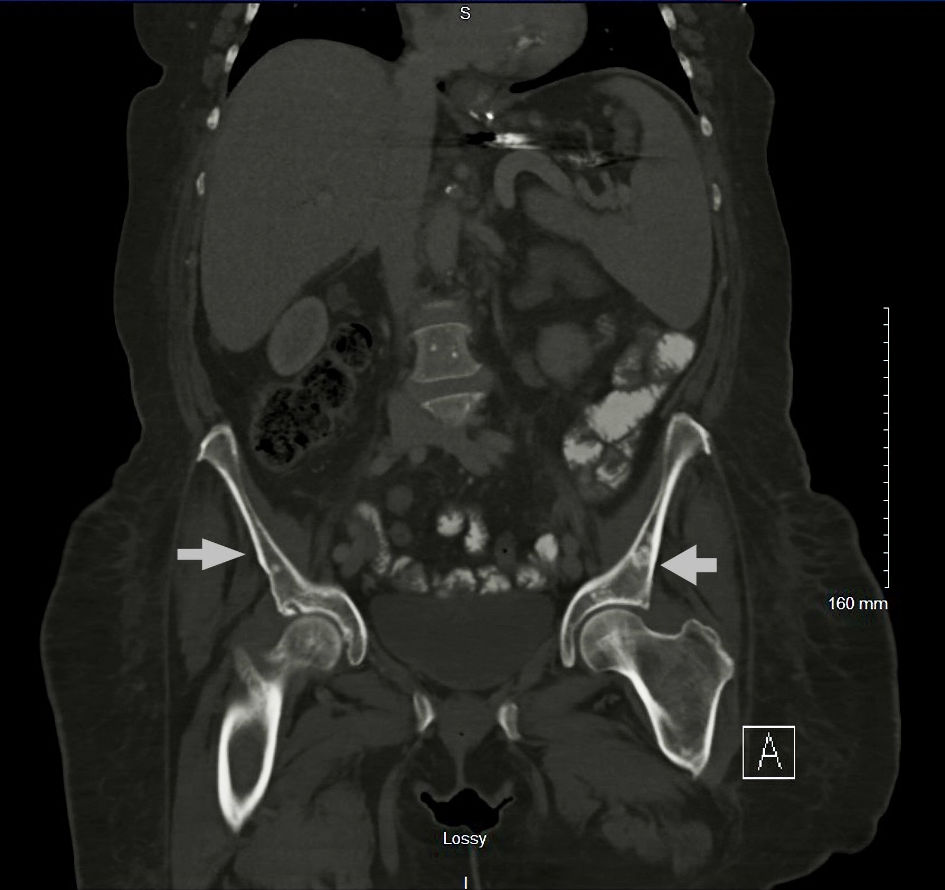 Click for large image | Figure 2. CT abdomen and pelvis with contrast showing the pelvic osteoblastic lesions (arrows). CT: computed tomography. |
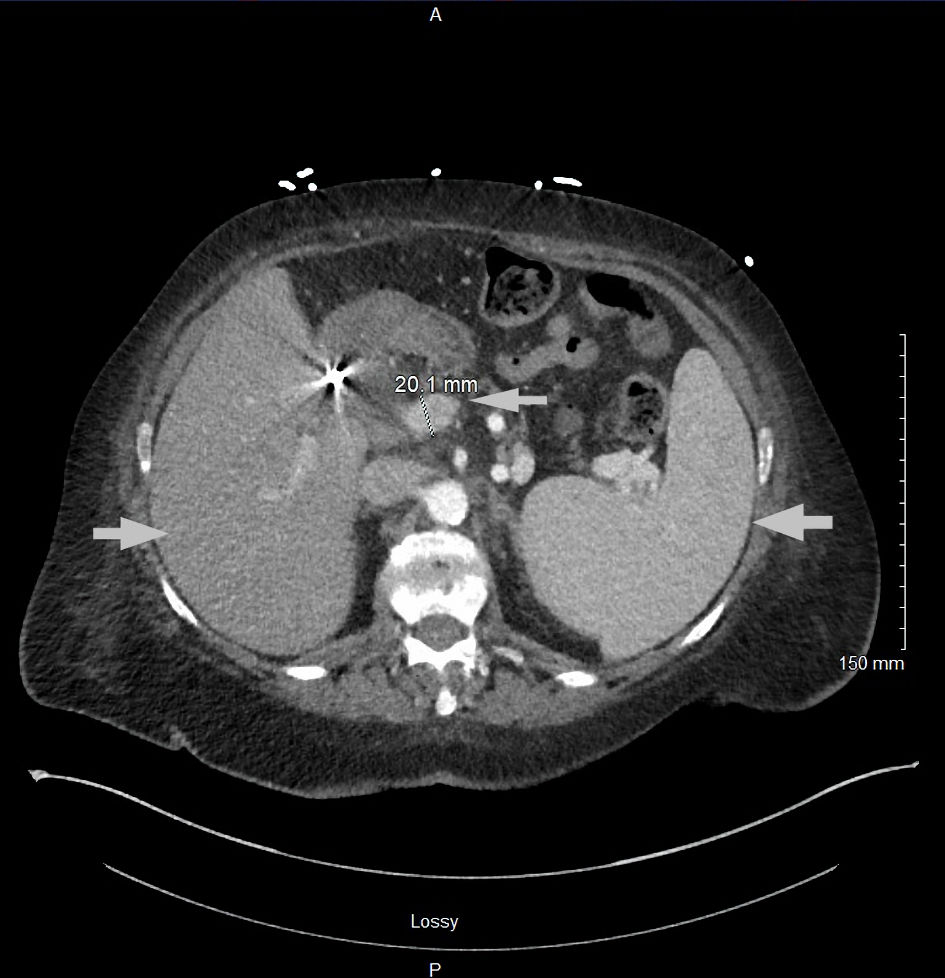 Click for large image | Figure 3. CT abdomen and pelvis with contrast showing hepatosplenomegaly and dilated portal vein (arrows). CT: computed tomography. |
The hematology-oncology team was consulted. The patient underwent a bone marrow biopsy to evaluate pancytopenia and due to suspicion of tumor infiltration of the bone marrow, as well as an interventional radiology-guided bone biopsy of left iliac sclerotic lesion. The bone marrow biopsy showed a hypercellular marrow for age with diffuse and patchy infiltration by large cells with fine basophilic granules, and microvacuoles most compatible with atypical mast cells (Fig. 4), with increased CD117 (Fig. 5) and tryptase positivity consistent with acute MCL. Flow cytometry also showed increased CD117-positive cells. These cells also expressed CD33, CD4 and CD13. Serum tryptase level was performed and was elevated at 526 µg/L (normal range ≤ 10.9 µg/L), further strengthening the diagnosis of MCL. The left ilium bone biopsy showed dense areas of atypical mast cell infiltrates (Fig. 6) and atypical mononuclear cells positive for CD117 (Fig. 7) and tryptase, compatible with MCL. Myeloid disorders mutation panel detected TP53 p.R273H mutation (tier 1 variant).
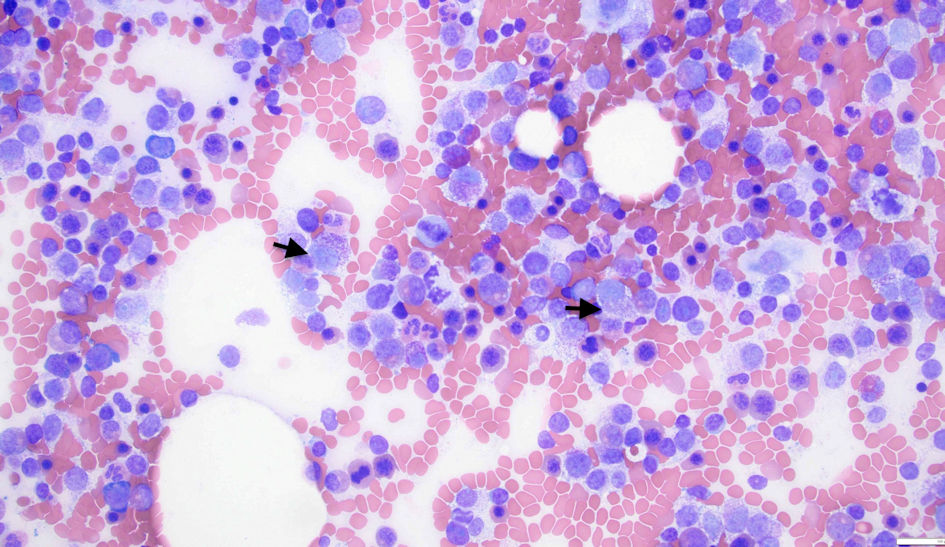 Click for large image | Figure 4. Bone marrow biopsy showing atypical mast cells (arrows). |
 Click for large image | Figure 5. Bone marrow biopsy showing cells with CD117 expression. |
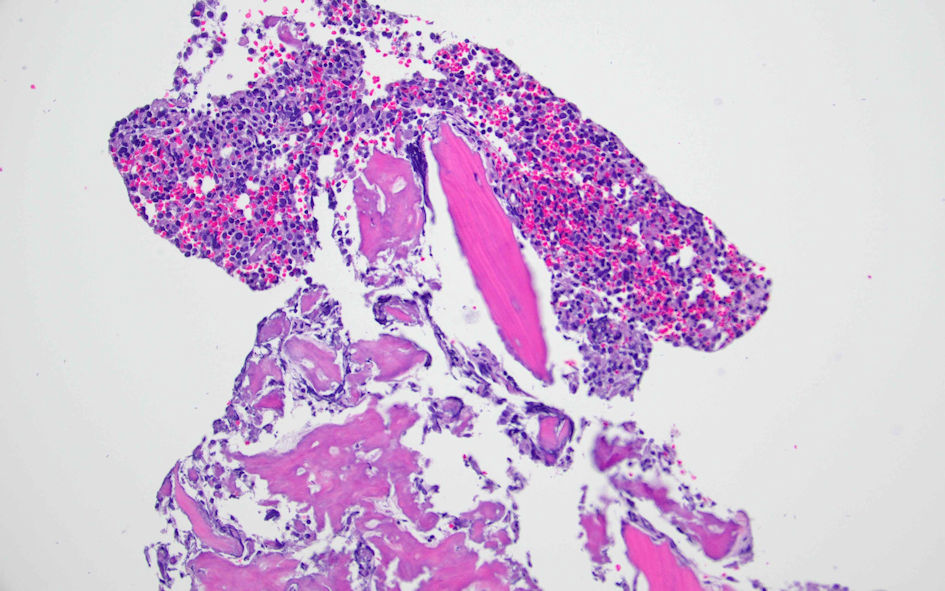 Click for large image | Figure 6. Left ilium bone biopsy showing atypical mast cells. |
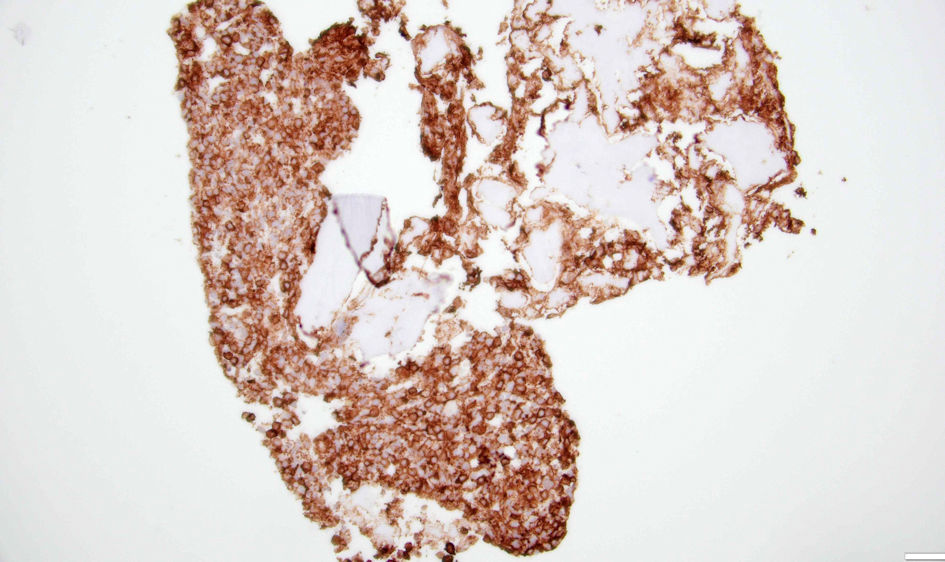 Click for large image | Figure 7. Left ilium bone biopsy showing cells with CD117 expression. |
Treatment with Food and Drug Administration (FDA)-approved agents (avapritinib or midostaurin), as well as transfer to a higher center with available clinical trials, was discussed. However, the patient developed acute metabolic encephalopathy with rapid clinical deterioration, and due to her declining functional status and overall poor prognosis, she was transitioned to hospice care. Patient expired under hospice care approximately 3 weeks after initial presentation.
| Discussion | ▴Top |
Mastocytosis is an abnormal accumulation of neoplastic mast cells in various organs, primarily divided into cutaneous mastocytosis (CM), SM, and mast cell sarcoma (MCS) [1, 2]. SM is further subdivided into six types: bone marrow mastocytosis (BMM), indolent SM (ISM), smoldering SM (SSM), aggressive SM (ASM), SM-associated hematologic neoplasm (SM-AHN) and MCL [1]. Advanced SM (AdvSM) is a subgroup that includes three subtypes of SM: ASM, SM-AHN, and MCL [1, 2].
MCL is the most aggressive form of SM that is diagnosed after meeting SM criteria and by the presence of ≥ 20% of atypical or immature mast cells in the bone marrow or ≥ 10% in the peripheral blood; however, an aleukemic variant is uncommonly seen, in which the number of circulating mast cells in the blood is < 10%, like our patient in this case [1-3, 5]. MCL is further classified into primary and secondary types: primary occurs de novo without prior mast cell disorders, and secondary arises from a preexisting mast cell neoplasm [2, 5]. MCL can be classified as acute or chronic. Acute MCL typically presents with C-findings indicating end-organ damage such as bone marrow dysfunction, skeletal and gastrointestinal manifestations [2, 3]. Chronic MCL presents without C-findings and usually has a better prognosis [2, 6].
Common clinical findings of MCL include constitutional symptoms (fever, fatigue, night sweats and weight loss), anemia, flushing, urticarial rash, gastrointestinal bleeding, skeletal pain, and diarrhea. The initial workup shows abnormal liver function tests, cytopenias, coagulopathy including disseminated intravascular coagulation (DIC), hepatosplenomegaly, peptic ulcer disease, and bone findings as described below [2-4]. Skin lesions are typically absent except in secondary MCL [2, 3]. Elevated serum tryptase levels further strengthen the diagnosis of MCL. The most common molecular abnormality observed in MCL is the KIT D816V mutation, which is present in approximately 90% of cases with MCL [2]. In a report of rare case of acute aleukemic MCL in a Hispanic male, authors reported most of the common clinical features (including DIC) and histopathological findings noted above [7]. Usual bone manifestations of MCL are osteoporosis and focal osteolytic lesions with pathological fractures [3, 4, 8]. In another case of acute aleukemic MCL, patient was reported to have a left humeral osteolytic lesion [9]. Osteoblastic (sclerotic) lesions are rarely seen in MCL. It is also observed that bone metastases in MCL outside the vertebral column are uncommon, and lesions beyond the axial skeleton are extremely rare [2, 4, 8].
Treatment options for SM, particularly MCL are limited, and there are multiple ongoing clinical trials targeting different treatment approaches [10, 11]. Treatment modalities with chemotherapeutic agents (such as cladribine, cytarabine, thalidomide, and daunorubicin) [10, 11], interferon-alpha (IFN alpha), glucocorticoids [12, 13], tyrosine kinase inhibitors, antibody drug conjugates, and allogenic hematopoietic stem cell transplantation (HSCT) [13, 14], are used with variable response. The two targeted therapies approved for MCL are midostaurin, a multikinase inhibitor, and avapritinib, a selective KIT D816V mutation-targeted tyrosine kinase inhibitor [10, 12, 13, 15]. Here, we review some of the pertinent studies and clinical trials.
In a multicentric phase II study, researchers reported that thalidomide is an effective treatment option in AdvSM when used in selected patients, but its use in MCL is not well studied [16]. A phase II trial by Gotlib et al played a pivotal role in the approval of midostaurin for AdvSM, including highly fatal variants of MCL [17]. In a phase IV clinical trial and literature review, authors reported that imatinib (a tyrosine kinase inhibitor that targets the break point cluster-Abelson tyrosine kinase (BCR-ABL)) was effective in SM patients without the KIT D816V mutation; however, imatinib was not effective in patients with the KIT D816V mutation [18]. A clinical trial designed to assess the efficacy of combination therapy using cladribine and pegylated IFN alfa-2a in patients with AdvSM was discontinued as the combo demonstrated no notable efficacy [19].
A multicenter trial studying inhalation cromolyn reported improvement in mast cell-related symptoms and overall quality of life, but the authors concluded that further clinical assessment in larger trials would be necessary to confirm the long-term benefits and safety of this approach [20]. The trial conducted by the National Heart, Lung, and Blood Institute (NHLBI) demonstrated that HSCT could be a feasible treatment option for patients with AdvSM, particularly in patients with AHN or aggressive systemic disease. This study contributed to the evolving knowledge of how HSCT could be used as a potential treatment for AdvSM [21]. A phase II clinical trial assessing ibrutinib (a Bruton’s tyrosine kinase (BTK) inhibitor) in patients with AdvSM was terminated due to no proven benefit [22]. In another phase II clinical trial, researchers concluded that they would not recommend brentuximab vedotin, an antibody drug conjugate, as monotherapy for patients with AdvSM [23].
Apex trial investigating bezuclastinib (a novel highly selective KIT D816V inhibitor) in AdvSM including MCL, is now actively recruiting patients [24]. Early data from this study showed that bezuclastinib was well tolerated with no serious adverse events, and there were notable reductions in disease markers [24]. Results from two active studies, Explorer phase I trial [25] and Pathfinder phase II trial [26] showed that the avapritinib has a good response and favorable benefit-risk profile in patients with AdvSM, irrespective of prior treatment or disease subtype. These studies led to the FDA approval of avapritinib in AdvSM including MCL. Pioneer study led to the FDA approval of avapritinib in ISM [27]. There is an active multicenter study that is evaluating elenestinib (another tyrosine kinase inhibitor that targets KIT D816V mutation) both as monotherapy and in combination with azacytidine, in patients with AdvSM and other KIT-altered hematologic malignancies [28].
Supportive measures also play a crucial role in the management of MCL during complications such as cytopenias, infections with sepsis, tumor lysis syndrome, mast cell activation syndrome (MCAS) and organ dysfunction [10-13]. MCL has overall survival (OS) ranging between 0 and 2.5 years, with median OS for all types of MCL being around 1.5 years. Acute MCL is highly aggressive and carries an extremely poor prognosis with median OS of 2 - 3 months despite treatment [6, 9, 14, 29]. In a case report of acute aleukemic MCL, it was reported that patient expired within the first 2 months of diagnosis despite high-dose chemotherapy [29].
Conclusions
We described here a very rare case of aleukemic MCL with diffuse osteoblastic metastatic bone lesions involving axial and appendicular skeleton. The osteoblastic presentation of MCL in this case is confirmed by bone biopsy. By this case we would like to report that MCL is a malignancy that can present with both osteolytic and osteoblastic bone lesions and stress the importance of bone biopsy in suspected cases of MCL. Mainstay of management of MCL includes targeted chemotherapies, midostaurin or avapritinib, and supportive measures. MCL carries a very poor prognosis, particularly acute MCL, which has median OS of 2 - 3 months despite treatment.
Learning points
MCL could present with both osteoclastic and osteoblastic metastatic lesions of the bone, and bone biopsy could play an important role in the diagnosis.
Extra-axial (appendicular) bone involvement of MCL is extremely rare, and no such cases were reported in the literature so far.
This case report adds to the limited data and information available in the literature on MCL clinical presentation and management.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Conflict of Interest
The authors declare that they do not have any conflict of interest.
Informed Consent
Patient expired before the case report is written. No patient identifiers or pictures of the patient are used in this manuscript. Verbal consent is obtained from the patient’s husband.
Author Contributions
All the authors were actively involved either in writing the different sections of the article, helping with images/ references, or revising the article prior to submission. Dr. Muralidhar Idamakanti (primary author) started the article/ report, wrote the manuscript with contributions in Abstract, Case Report, Discussion, and literature search. Dr. Ala Ebaid contributed to the article design, writing and editing of the manuscript, and literature search. Dr. Rani Indrani Bijjam helped with patient’s history and writing it in the report, literature search and CT images. Dr. Shivakumar Mukkamala identified the rareness of the case report and contributed to writing the case report and editing the manuscript. Dr. Alexei Bakhirev helped with the histopathological section of the case report and obtained the pathology slides. Dr. Leslie Andritsos helped with editing the manuscript and provided valuable input on management and the article’s conclusions.
Data Availability
The authors declare that data supporting the findings of this case report are available within the article.
Abbreviations
MCL: mast cell leukemia; SM: systemic mastocytosis; BMI: body mass index; CT: computed tomography; CD: cluster differentiation; FDA: Food and Drug Administration; CM: cutaneous mastocytosis; MCS: mast cell sarcoma; BMM: bone marrow mastocytosis; ISM: indolent systemic mastocytosis; SSM: smoldering systemic mastocytosis; ASM: aggressive systemic mastocytosis; AHN: associated hematologic neoplasm; AdvSM: advanced systemic mastocytosis; DIC: disseminated intravascular coagulation; IFN: interferon; BCR-ABL: break point cluster-Abelson tyrosine kinase; HSCT: hematopoietic stem cell transplantation; NHLBI: National Heart, Lung, and Blood Institute; BTK: Bruton’s tyrosine kinase; OS: overall survival
| References | ▴Top |
- Nahla AM H. The 2023 updated classification and diagnostic criteria of mastocytosis. Canc Therapy & Oncol Int J. 2023;24(4):556141.
doi - Zanelli M, Quintini M, Magnasco S, Aprile L, Palicelli A, Zizzo M, Sanguedolce F, et al. Mast cell leukemia: an update with a practical review. Cancers (Basel). 2023;15(6):1664.
doi pubmed - Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285-1295.
doi pubmed - King JJ, Crawford EA, Iwenofu OH, Fox EJ. Case report: pathologic long bone fracture in a patient with systemic mastocytosis. Clin Orthop Relat Res. 2007;459:263-269.
doi pubmed - Horny HP, Krokowski M, Feller AC, Hintze G, Sotlar K, Valent P. [Aleukemic mast cell leukemia (formerly: "malignant mastocytosis"): an extremely rare form of leukemia. A case report and simultaneously a contribution to revised classification of mastocytosis]. Wien Klin Wochenschr. 2002;114(5-6):222-228.
pubmed - Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, McClure RF, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727-5736.
doi pubmed - Galura GM, Cherukuri SV, Hakim N, Gaur S, Orazi A. Acute aleukemic mast cell leukemia: Report of a case and review of the literature. Leuk Res Rep. 2020;14:100230.
doi pubmed - Rossini M, Zanotti R, Viapiana O, Tripi G, Orsolini G, Idolazzi L, Bonadonna P, et al. Bone involvement and osteoporosis in mastocytosis. Immunol Allergy Clin North Am. 2014;34(2):383-396.
doi pubmed - Ender E, Dages K, Pitlick M, Voelker D, Pongdee T. Mast cell leukemia: a case report. Annals of Allergy, Asthma & Immunology. 2022;129(5):S164.
- Gilreath JA, Tchertanov L, Deininger MW. Novel approaches to treating advanced systemic mastocytosis. Clin Pharmacol. 2019;11:77-92.
doi pubmed - Helbig G, Koclega A, Gawel WB, Wlodarczyk M, Rodzaj M, Labedz A, Hus I, et al. The efficacy of cladribine (2-CdA) in advanced systemic mastocytosis. Indian J Hematol Blood Transfus. 2020;36(4):661-666.
doi pubmed - Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, Bennett JM, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003;27(7):635-641.
doi pubmed - Lee HJ. Recent advances in diagnosis and therapy in systemic mastocytosis. Blood Res. 2023;58(S1):96-108.
doi pubmed - Bauer J, Longo W, Yang D. Mast cell leukemia: review of a rare disease and case report of prolonged survival after allogeneic stem cell. Transplant Hum Pathol Case Rep. 2017;10:46-49.
doi - Piris-Villaespesa M, Alvarez-Twose I. Systemic mastocytosis: following the tyrosine kinase inhibition roadmap. Front Pharmacol. 2020;11:443.
doi pubmed - Gruson B, Lortholary O, Canioni D, Chandesris O, Lanternier F, Bruneau J, Grosbois B, et al. Thalidomide in systemic mastocytosis: results from an open-label, multicentre, phase II study. Br J Haematol. 2013;161(3):434-442.
doi pubmed - Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, Awan FT, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541.
doi pubmed - Alvarez-Twose I, Matito A, Morgado JM, Sanchez-Munoz L, Jara-Acevedo M, Garcia-Montero A, Mayado A, et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget. 2017;8(40):68950-68963.
doi pubmed - Cladribine plus pegylated interpheron Alfa-2a in systemic mastocytosis. NCT01602939. August, 2016. https://clinicaltrials.gov/study/NCT01602939.
- Treatment of indolent systemic mastocytosis with PA101. NCT02478957. 2016. https://clinicaltrials.gov/study/NCT02478957.
- Stem cell transplantation to treat systemic mastocytosis. NCT00006413. 2017. https://clinicaltrials.gov/study/NCT00006413.
- Ibrutinib in treating patients with advanced systemic mastocytosis. NCT02415608. 2018. https://clinicaltrials.gov/study/NCT02415608.
- Gotlib J, Baird JH, George TI, Langford C, Reyes I, Abuel J, Perkins C, et al. A phase 2 study of brentuximab vedotin in patients with CD30-positive advanced systemic mastocytosis. Blood Adv. 2019;3(15):2264-2271.
doi pubmed - DeAngelo DJ, Pullarkat V, Piris-Villaespesa M, George TI, Patel JL, Ustun C, et al. P1049: a phase 2 study of bezuclastinib (CGT9486), a novel, highly selective, potent KIT D816V inhibitor, in adults with advanced systemic mastocytosis (APEX): methods, baseline data, and early insights. Hemasphere. 2022;6(Suppl):939-940.
doi - DeAngelo DJ, Radia DH, George TI, Robinson WA, Quiery AT, Drummond MW, Bose P, et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial. Nat Med. 2021;27(12):2183-2191.
doi pubmed - Gotlib J, Reiter A, Radia D, Deininger M, George T, Panse J, et al. P1023: avapritinib in patients with advanced systemic mastocytosis (ADVSM): efficacy and safety analyses from the phase 2 pathfinder study with 2-year follow-up. Hemasphere. 2023;7(Suppl):e3184876.
doi - Gotlib J, Castells M, Elberink HO, Siebenhaar F, Hartmann K, Broesby-Olsen S, George TI, et al. Avapritinib versus placebo in indolent systemic mastocytosis. NEJM Evid. 2023;2(6):EVIDoa2200339.
doi pubmed - A phase 1/2 study of Elenestinib (BLU-263) as monotherapy and in combination with azacitidine in patients with advanced systemic mastocytosis (AdvSM) and and other KIT altered hematologic malignancies. NCT05609942. 2024. https://clinicaltrials.gov/study/NCT05609942.
- Zeerleder S, van Oers M. Aleukemic variant of mast cell leukemia. Blood. 2012;119(9):1961.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.