| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 000, Number 000, April 2025, pages 000-000
A Novel Therapeutic Strategy for Central Nervous System Lymphoma: Integrating Chimeric Antigen Receptor T-Cell Therapy and Gamma Knife Radiation
Katherine Hickmanna, Rachel DiLeob, Kathleen Faringerc, Chelsea Petersonc, Rodney Wegnerd, Zachary Horned, Yazan Samhouric, e
aDrexel University College of Medicine, Philadelphia, PA, USA
bDepartment of Internal Medicine, Allegheny Health Network, Pittsburgh, PA, USA
cDivision of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA
dDivision of Radiation Oncology, Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA
eCorresponding Author: Yazan Samhouri, Division of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA 15224, USA
Manuscript submitted January 16, 2025, accepted March 28, 2025, published online April 11, 2025
Short title: CAR T and GK-SRS in Refractory SCNSL
doi: https://doi.org/10.14740/jh2029
| Abstract | ▴Top |
Central nervous system lymphoma (CNSL) is an aggressive disease with limited well-studied options for treatment, especially refractory treatment. First-line treatment usually includes high-dose methotrexate (HD-MTX) for induction and either autologous stem cell transplantation or whole-brain radiation therapy (WBRT) as consolidation. However, WBRT can result in significant neurotoxicity, so the use of focal radiation (i.e., gamma knife-stereotactic radiosurgery (GK-SRS)) of varying doses and fractions has been proposed. In the case of refractory disease, chimeric antigen receptor (CAR) T-cell therapy has begun to be used clinically, but patients with CNS involvement were left out of key approval trials. Here, we present a case of a 62-year-old patient with refractory secondary CNSL (SCNSL) previously treated with WBRT who was successfully treated with a combination of CAR T-cell therapy and GK-SRS.
Keywords: CAR T-cell; GK-SRS; CNSL
| Introduction | ▴Top |
Central nervous system lymphoma (CNSL), encompassing both primary (PCNSL) and secondary (SCNSL) forms, presents a formidable challenge due to its aggressive nature and dismal prognosis [1]. Current standard-of-care for CNSL relies heavily on high-dose methotrexate (HD-MTX)-based regimens for induction, followed by consolidation strategies involving high-dose chemotherapy plus autologous stem cell transplantation (ASCT), or whole-brain radiation therapy (WBRT) [2, 3]. When radiation is used, WBRT is preferred over focal radiation, given the infiltrative nature of the disease, but it comes with significant neurotoxicity risk due to damage to healthy brain tissue, posing a considerable clinical challenge [2].
Despite these efforts, a substantial proportion of patients exhibit resistance to first-line therapies, necessitating the exploration of alternative treatment approaches. Second-line strategies have included high-dose chemotherapy, ibrutinib, lenalidomide, checkpoint inhibitors, and other small molecules, either as monotherapy or in combination [4, 5]. However, response rates with these agents are often low, and responses are frequently transient.
The advent of chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment landscape of B-cell malignancies, expanding into diverse applications since its approval for diffuse large B-cell lymphoma (DLBCL) in 2017 [5]. Initially, concerns regarding the potential for neurotoxicity led to the exclusion of patients with CNS disease involvement in early clinical trials. Additionally, there is always a concern in CNS disease that therapies will not effectively penetrate the blood-brain barrier (BBB). Despite these concerns, emerging evidence suggests that CAR T-cell therapy holds promise for CNSL patients with effective BBB penetration and minimal neurotoxicity [6].
This case report presents a compelling example of a 62-year-old patient with refractory SCNSL who achieved a successful response through a combination of CAR T-cell therapy and gamma knife-stereotactic radiosurgery (GK-SRS).
| Case Report | ▴Top |
A 62-year-old Hispanic male presented with a history of DLBCL, ABC subtype, initially diagnosed in 2020. The primary tumor was located in the left tonsillar region, with additional disease in the left cervical region and omentum. He received six cycles of R2CHOP (rituximab, cyclophosphamide, vincristine, doxorubicin, prednisone, and lenalidomide) achieving a complete response (CR) as documented by positron emission tomography (PET)/computed tomography (CT). Notably, his Central Nervous System International Prognostic Index (CNS-IPI) score was low, rendering CNS prophylaxis unnecessary at that time.
Around 2 years after initial diagnosis, the patient was hospitalized due to altered mental status. Brain magnetic resonance imaging (MRI) revealed diffusely infiltrative lesions involving the deep structures of the right brain, extending into the left side and the pituitary stalk (Fig. 1). His mental status deteriorated, with repeat CT scans showing concern for midline shift and early uncal herniation, leading to intubation and placement of an external ventricular drain (EVD). A brain biopsy confirmed DLBCL ABC subtype, consistent with SCNSL. There was no evidence of systemic relapse.
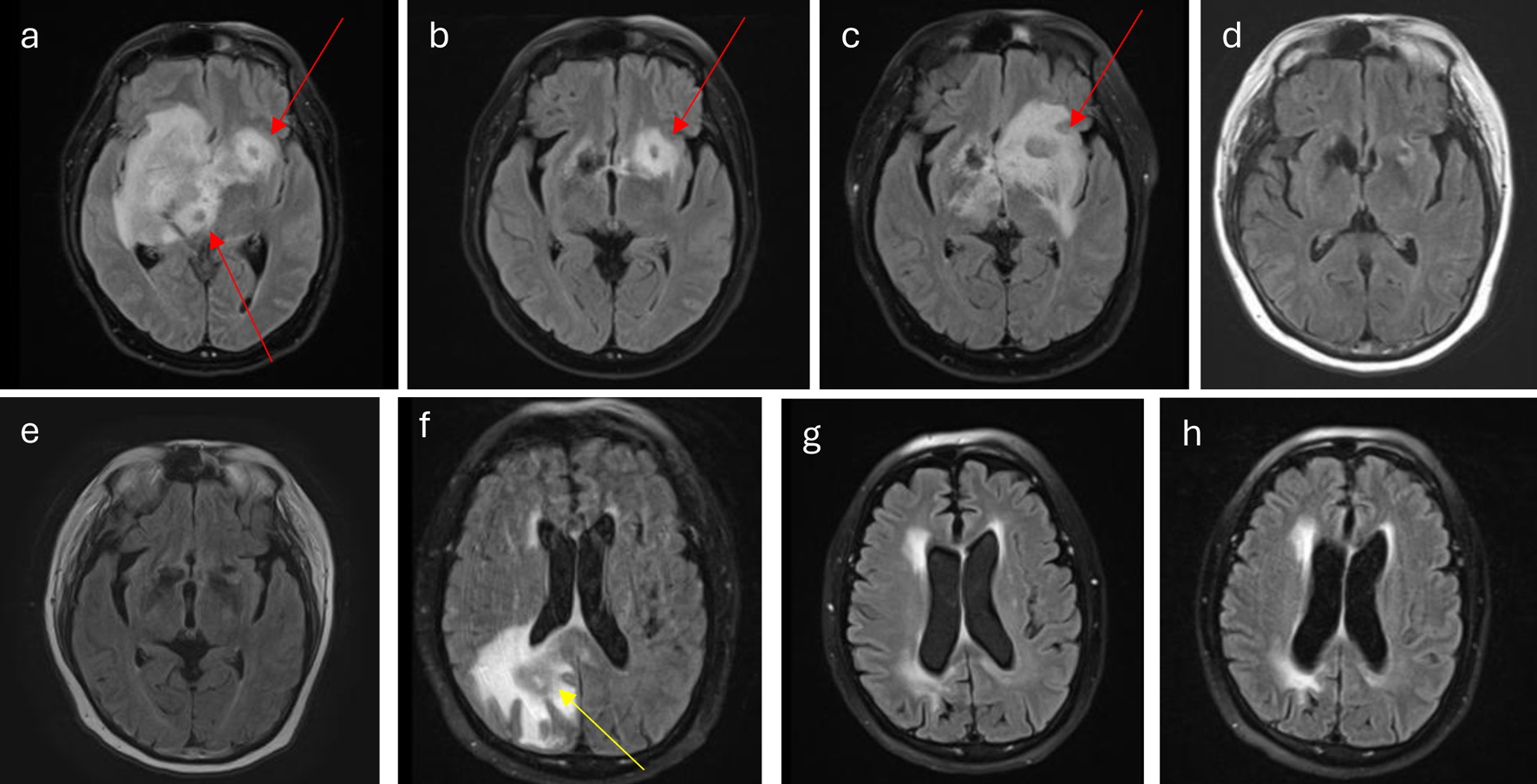 Click for large image | Figure 1. (a) At diagnosis, lesions (red arrows) involve right caudate thalamic groove, thalamus, basal ganglia, corona radiata, inferior medial frontal lobe, medial temporal lobe, the midbrain, and pons. There is also extension into the corpus callosum, septum pellucidum, fornix, hypothalamus, right optic chiasm, left caudate thalamic groove, inferior frontal lobe, and pituitary stalk. (b) Interval decrease of lesion (red arrow) during acalabrutinib and Temodar treatment. (c) Worsening of disease burden (red arrow) at the end of acalabrutinib and Temodar treatment. (d) CR after four cycles of HD-MTX plus rituxan and cytarabine. (e) Continuing CR after WBRT. (f) Disease relapse (yellow arrow) 2 months from (e). (g) CR following CAR T-cell therapy. (h) Continued CR following GK-SRS. CAR: chimeric antigen receptor; CR: complete response; GK-SRS: gamma knife-stereotactic radiosurgery; HD-MTX: high-dose methotrexate; WBRT: whole-brain radiation therapy. |
Treatment for SCNSL commenced with HD-MTX, but this was complicated by acute kidney injury necessitating glucarpidase administration and hypernatremia secondary to diabetes insipidus, requiring desmopressin (DDAVP) therapy. Due to HD-MTX intolerance, the patient was switched to acalabrutinib and temozolomide in the subsequent cycle. While a mid-treatment scan showed initial improvement, subsequent imaging a month later revealed worsening CNSL with no evidence of systemic disease (Fig. 1).
The patient was then re-challenged with a combination of HD-MTX, reduced-dose (rd) cytarabine, and rituximab for four cycles. This regimen resulted in a good response after two cycles, culminating in a CR after four cycles (Fig. 1). However, due to comorbidities and performance status, the patient was deemed ineligible for ASCT. Subsequently, he underwent WBRT receiving 13 fractions of 180 cGy, totaling 2,340 cGy. Follow-up imaging at a year after initial relapse demonstrated continued CR (Fig. 1), leading to the initiation of temozolomide maintenance therapy.
Unfortunately, a brain MRI 2 months after CR revealed recurrent, bulky disease (Fig. 1). With the patient now refractory to multiple lines of therapy, a decision was made to pursue CAR T-cell therapy. The implications of off-label CAR T-cell therapy, given the original exclusion of CNS disease patients in DLBCL trials, were thoroughly discussed with the patient, and informed consent was obtained. As bridging therapy, he received two cycles of HD-MTX with procarbazine and vinblastine prior to CAR T-cell infusion.
The patient received axicabtagene ciloleucel infusion as planned and experienced no significant cytokine release syndrome (CRS) or immune effector cell-associated neurotoxicity syndrome (ICANS) beyond his neurological baseline. A post-infusion MRI revealed a good response to CAR T-cell therapy, with decreased size of enhancement to around 2 cm, and decreased mass effect and vasogenic edema. This remnant enhancement may represent pseudoinflammation or remnant mass following CAR T-cell therapy. However, due to the significant disease burden, with mass measuring 5.2 cm in size at its largest, he was deemed high-risk for relapse. Consequently, after multidisciplinary discussion, the patient was referred to radiation oncology to pursue GK-SRS in addition to CAR T-cell therapy. He received a single fraction of 1,200 cGy to the area of concern 30 days following CAR T-cell infusion. At this time, the enhancement was 2 cm in size, but treatment volume was based on tumor bed prior to CAR T-cell infusion.
Since then, the patient has been doing well, with no evidence of disease progression on repeat imaging 1 year since CAR T-cell therapy administration.
| Discussion | ▴Top |
CAR T-cell therapy has been approved for relapsed/refractory DLBCL, but due to original concern for increased risk of neurotoxicity, those with active CNS disease were excluded from the approval. However, after it has entered the market, patients with CNS disease have received treatment with no increased neurotoxicity [5]. The accepted hypothesis now is that the neurotoxicity associated with CAR T-cell therapy, known more broadly as ICANS, is related more to the systemic inflammation produced from the T-cell proliferation than underlying neurological disease. In theory, those with only CNS disease are at less risk for this increased inflammatory response due to the lack of systemic disease. Another concern with any therapy used in CNSL is the penetration of the BBB. However, CAR T cells have been found in the cerebrospinal fluid (CSF) up to 6 months after the infusion, showing good penetration [1]. The decision to pursue CAR T-cell therapy in our patient at the time of treatment was based on promising results from nine case reports utilizing the therapy in the relapsed/refractory setting published by Dana-Farber in Jacobson et al [7]. Additionally, in a meta-analysis of 128 patients with CNSL treated with CAR T-cell therapy, Cook et al found an overall CR rate of 56% and 47% for PCNSL and SCNSL, respectively, which is comparable to rates in systemic large B-cell lymphoma [5]. These point toward a role for CAR T-cell therapy in relapsed/refractory CNSL; however, there are still concerns about how to handle combination therapy for patients with particularly high burden of disease or partial response after therapy. Our patient was considered high risk due to the size of their lesion being greater than 5 cm, which has been recently shown in DLBCL after CAR T-cell therapy to be at high risk of local failure [8].
Radiation therapy for the treatment of lymphoma is fundamentally different from other CNS tumors due to the infiltrating nature of the disease. This is why although GK-SRS has replaced WBRT in the treatment scheme of solid tumor metastasis to the CNS, GK-SRS is not commonplace and WBRT is still the mainstay of treatment for CNSL [2]. Additionally, since WBRT must be a lower dose than targeted therapy to avoid neurotoxic affects, this trades off the chance of better local control that focal radiation could provide, which may be significant in patients at high risk of relapse like our patient who had already progressed through standard care. However, in the case of relapsed/refractory patients who have received WBRT or were not considered candidates for WBRT, there are limited options. One option beginning to be explored in these situations is focal radiation (i.e., GK-SRS) [9].
Our patient was treated with one dose of 12 Gy SRS boost. This regimen was based on Foreman et al which included a retrospective study from 2008 to 2021 of patients who received a GK-SRS boost following a previous rd-WBRT, like our patient who had received rd-WBRT a year prior [10]. However, GK-SRS is only one form of focal radiation, as there is also hypofractionated volumetric modulated arc therapy (VMAT)/intensity-modulated radiation therapy (IMRT), linear accelerator SRS, and GK-SRS. Additionally, both SRS and GK-SRS within small studies have been quite successful in achieving local control at the site of radiation in CNSL, with reports detailing close to 100% local control after treatment [2, 9, 11, 12]. Arc-based radiation like VMAT/IMRT, although successful in non-CNS lesions, was not considered in our patient because less precision control posed increased risk to healthy brain tissue that had already received WBRT, although it has been considered as an option in multiple brain metastasis and may be considered in the future [13]. GK-SRS was chosen over linear accelerator SRS in our patient partially due to the above study, the increased precision that the gamma knife machine provides, and need for only one fraction [10]. However, the issue that has arisen in attempts to use SRS alone in CNSL specifically is the rates of distant disease recurrence. This indicates a need for additional systemic therapy to achieve more durable remission.
One approach in combining treatment modalities to treat high-risk relapsed/refractory CNSL includes using focal radiation as a type of bridging therapy as CAR T-cell production takes time. This approach has been explored in Cederquist et al and included both WBRT and SRS as bridging [14]. However, as we explored in our patient’s case, another approach is using CAR T-cell therapy as induction and GK-SRS as a type of consolidation therapy. One concern in deciding to pursue post CAR T-cell radiation in our patient and in general, is if the radiation will decrease the efficacy by killing the CAR T cells at the tumor site. However, in our patient’s case, a significant interval decrease had been demonstrated at the day +30 time of radiation post CAR T-cell therapy. Additionally, based on the noteworthy local control of SRS discussed above even if there is some loss of cells, their presence at distant microscopic sites is likely more important in long-term progression-free survival than imaged tumor bed site where radiation should provide control [2, 9, 11, 12]. However, although radiation at day +30 provided a CR and continued progression-free survival for this patient, more studies are needed to determine the best timing of pursuing focal radiation post CAR T-cell treatment to achieve the most durable remission.
The addition of CAR T-cell therapy to GK-SRS will hopefully decrease the risk of distant recurrence after treatment. Additionally, in patients with large disease burden, have previously received WBRT, or have partial response to CAR T-cell therapy, GK-SRS offers better local control with minimal toxicity compared to WBRT. We present this case as proof of concept and to add to the literature about its safety in the hope that this case offers a path of possible treatment and remission for those with high-risk disease with less risk of toxicity. We recognize our patient is only one case, so further exploration in the future is needed to improve long-term outcomes studies utilizing CAR T-cell therapy with GK-SRS.
Conclusion
With this example of the successful use of CAR T-cell therapy induction followed by GK-SRS consolidation in relapsed SCNSL, we hope our case demonstrates feasibility and safety of this therapy. Further research is needed to determine the best approach to administering these therapies in tandem and which patients are the best candidates for this to improve long-term patient outcomes.
Acknowledgments
None to declare.
Financial Disclosure
This case report was not funded.
Conflict of Interest
All authors have no conflict of interest.
Informed Consent
Informed consent was obtained for all treatment, and specifically for the use of CAR T-cell therapy the patient was informed that this would be an off-label use of this treatment but is approved for similar indication and has been used in similar scenarios since approval.
Author Contributions
Yazan Samhouri MD had full access to all the data and analysis in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. Concept and design: Yazan Samhouri MD. Drafting of the manuscript: all authors. Critical revision of the manuscript: all authors. Statistical analysis: N/A. Administrative and technical support: Yazan Samhouri MD. Supervision: Yazan Samhouri MD.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ASCT: autologous stem cell transplant; BBB: blood-brain barrier; CAR: chimeric antigen receptor; CR: complete response; GK-SRS: gamma knife-stereotactic radiosurgery; HD-MTX: high-dose methotrexate; PCNSL: primary central nervous system lymphoma; R2CHOP: rituximab, cyclophosphamide, vincristine, doxorubicin, prednisone, and lenalidomide; SCNSL: secondary central nervous system lymphoma; WBRT: whole-brain radiation therapy
| References | ▴Top |
- Cook MR, Dorris CS, Makambi KH, Luo Y, Munshi PN, Donato M, Rowley S, et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients. Blood Adv. 2023;7(1):32-39.
doi pubmed - Palmer JD, Bhamidipati D, Shukla G, Epperla N, Glass J, Kim L, Shi W. Outcomes after stereotactic radiosurgery for CNS lymphoma. J Neurooncol. 2020;147(2):465-476.
doi pubmed - Samhouri Y, Mustafa Ali MK, Law J, Khan C, Wegner R, Lee ST, Lister J. Consolidative autologous stem cell transplantation versus whole brain radiation in PCNSL; a nationwide analysis. Clin Lymphoma Myeloma Leuk. 2022;22(10):735-743.
doi pubmed - Ferreri AJM, Doorduijn JK, Re A, Cabras MG, Smith J, Ilariucci F, Luppi M, et al. MATRix-RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): an international, single-arm, phase 2 trial. Lancet Haematol. 2021;8(2):e110-e121.
doi pubmed - Frigault MJ, Dietrich J, Gallagher K, Roschewski M, Jordan JT, Forst D, Plotkin SR, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022;139(15):2306-2315.
doi pubmed - Karschnia P, Arrillaga-Romany IC, Eichler A, Forst DA, Gerstner E, Jordan JT, Ly I, et al. Neurotoxicity and management of primary and secondary central nervous system lymphoma after adoptive immunotherapy with CD19-directed chimeric antigen receptor T-cells. Neuro Oncol. 2023;25(12):2239-2249.
doi pubmed - Jacobson CA, Falvey C, Bouvier R, et al. A pilot study of axicabtagene ciloleucel (axi-cel) for the Treatment of Relapsed/Refractory Primary and Secondary Central Nervous System Lymphoma (CNSL). Blood 2022;140(Supplement 1):1060-61.
doi - Figura NB, Robinson TJ, Sim AJ, Wang X, Cao B, Chavez JC, Shah BD, et al. Patterns and predictors of failure in recurrent or refractory large B-cell lymphomas after chimeric antigen receptor T-cell therapy. Int J Radiat Oncol Biol Phys. 2021;111(5):1145-1154.
doi pubmed - Alvarez-Pinzon AM, Wolf A, Valerio JE, Borro M, Herrera D, Alonso JR. Gamma knife stereotactic radiosurgery as an effective tool in primary CNS lymphoma: Evaluation of stereotactic radiosurgery and methotrexate treatment in a prospective and observational clinical research study. Clin Neurol Neurosurg. 2021;201:106457.
doi pubmed - Foreman BE, Mullikin TC, Floyd SR, Kelsey CR, Patel MP, Peters KB, Kirkpatrick JP, et al. Long-term outcomes with reduced-dose whole-brain radiotherapy and a stereotactic radiosurgery boost for primary central nervous system lymphoma. Neurooncol Adv. 2023;5(1):vdad097.
doi pubmed - Schep DG, Mir T, Fraser GAM, Greenspoon JN. Fractionated stereotactic radiation for central nervous system lymphoma: retrospective analysis of initial cases. Curr Oncol. 2023;30(9):8602-8611.
doi pubmed - Wu SY, Braunstein SE, Rubenstein JL, Sneed PK. Stereotactic radiosurgery for primary central nervous system lymphoma. Cureus. 2023;15(2):e34817.
doi pubmed - Thomas EM, Popple RA, Wu X, Clark GM, Markert JM, Guthrie BL, Yuan Y, et al. Comparison of plan quality and delivery time between volumetric arc therapy (RapidArc) and Gamma Knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75(4):409-417; discussion 417-408.
doi pubmed - Cederquist GY, Schefflein J, Devlin SM, Shah GL, Shouval R, Hubbeling H, Tringale K, et al. CNS bridging radiotherapy achieves rapid cytoreduction before CAR T-cell therapy for aggressive B-cell lymphomas. Blood Adv. 2024;8(19):5192-5199.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.