| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Letter to the Editor
Volume 000, Number 000, December 2024, pages 000-000
Venetoclax-Azacitidine Salvage Chemotherapy in Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Single-Center Experience
Dina Osmana, b , Maria Losa Marotoa, Francesca Kinsellaa, Vidhya Murthya
aClinical Haematology Department, University Hospital Birmingham, Birmingham, UK
bCorresponding Author: Dina Osman, Queen Elizabeth Hospital, Birmingham B15 2GW, UK
Manuscript submitted October 9, 2024, accepted November 15, 2024, published online December 31, 2024
Short title: Venetoclax-Azacitidine in R/R AML
doi: https://doi.org/10.14740/jh1362
| To the Editor | ▴Top |
Acute myeloid leukemia (AML) is a malignant bone marrow disorder arising from early myeloid progenitors leading to clonal expansion and myeloid maturation arrest [1]. It is the commonest acute leukemia in adults with a rising incidence with age. The median age of diagnosis is 69 years, and the estimated age-adjusted annual incidence is 4.3/100,000 per year in the United States [1, 2]. The management of AML is rapidly evolving and the use of the BCL2 inhibitor, venetoclax in combination with azacitidine in previously untreated AML has revolutionized the management particularly in elderly co-morbid patients ineligible for intensive chemotherapy. The VIALE-A trial showed an overall survival (OS) advantage of 14.7 months compared to azacitidine monotherapy in previously untreated AML patients [3].
We assessed the response to venetoclax-azacitidine in a small cohort of patients with relapsed/refectory (R/R) AML and high-risk myelodysplastic syndrome (MDS) and the results were overall promising as this regimen could potentially be consolidated with stem cell transplant (SCT) or to donor lymphocyte infusion (DLI) in patients who previously had an SCT. Venetoclax-azacitidine can be mostly delivered in an outpatient setting, thus significantly reducing inpatient bed days and transfusion requirements compared to intensive salvage chemotherapy in selected patient cohort.
R/R AML has been a challenging entity for decades with a 5-year OS of approximately 10-20%. In the absence of targeted molecular therapy, conventional cytotoxic, high-dose cytarabine-based chemotherapy has been the gold standard induction therapy with allogeneic SCT representing the preferred consolidation strategy [4]. The combination of venetoclax and azacitidine is increasingly being used as a salvage therapy in R/R AML as a bridge to either SCT or DLI. However, the evidence available so far is obtained from small, single-arm studies and retrospective case series emphasizing the importance of running well-conducted randomized prospective clinical trials to obtain better-quality evidence [5].
A phase 2 study performed by Bazinet et al assessed the role of oral decitabine/cedazuridine with venetoclax in R/R AML and showed an overall response rate (ORR) of 50% after a median follow-up of 12.8 months [6]. The median OS was 7.6 months, with a median duration of response (DOR) of 4.6 months [6]. DiNardo et al performed a retrospective analysis of 39 R/R AML patients and two patients with MDS and blastic plasmacytoid dendritic cell neoplasm (BPDCN) treated with venetoclax and hypomethylating agent (HMA) or low-dose cytarabine (LDAC) [7]. The median OS was 3 months for the whole cohort and 4.8 months for responding patients, with better ORR seen in IDH1/2 and RUNX1-mutated patients with 27% and 50%, respectively [7].
In a propensity score matching analysis performed by Unglaub et al, 37 patients with R/R AML who received venetoclax-based combination chemotherapy were compared to 90 patients from the German Study Alliance Leukemia who were treated according to the physician’s personal choice with non-venetoclax-based chemotherapy [8]. The ORR and the median event-free survival (EFS) were significantly higher in the venetoclax group (62% vs. 42%; P = 0.049 and 8.0 months vs. 3.7 months; P = 0.006, respectively). The median OS was not superior in the venetoclax-treated group. In a cohort of 43 R/R AML patients treated with venetoclax-based combination therapy, the ORR was 76.2% and the OS was 9.3 months [9].
We retrospectively analyzed the response of 15 patients with R/R AML and four patients with high-risk MDS treated with venetoclax-azacitidine as salvage therapy from September 2021 to February 2023 at the Queen Elizabeth Hospital Birmingham. The baseline characteristics of the study population can be seen in Table 1. As per the four-gene expressor European LeukemiaNet (ELN) 2024 risk stratification [10], 10 patients (66.6%) had favorable risk disease, two (13.3%) had intermediate risk, and three (20%) had adverse risk disease. Prior to venetoclax-azacitidine chemotherapy, 12 AML patients (80%) received one line of chemotherapy, whereas three patients (20%) had two lines including FLAG-Ida salvage. Two MDS patients (50%) achieved remission with azacitidine, and one (25%) required FLAG-Ida salvage. No patients were previously exposed to venetoclax. The median number of delivered venetoclax-azacitidine cycles was 2. Four patients (21%) received 28 days of venetoclax, and the remaining patients had 14 - 21 days. Almost all patients developed at least grade 3 neutropenia, and 10 patients (52.6%) were admitted with neutropenic fevers during or after the first cycle. Red cell and platelet transfusion support were required in 13 (68.4%) and eight (42.1%) patients, respectively, with a median of seven red cell units (range 1 - 16) and 11 platelet units (range 2 - 23).
 Click to view | Table 1. Baseline Characteristics of the Study Population |
Fifteen patients (78.9%) achieved complete remission (CR/CRi) post venetoclax-azacitidine chemotherapy, including the NPM1-mutated patients who achieved molecular remission. Seven patients (36.8%) underwent consolidation with allogeneic SCT and six patients (31.5%) received DLI. One patient (5.2%) had detectable molecular disease after two cycles and was refractory to gilteritinib. Four patients (20%) were refractory to venetoclax-azacitidine chemotherapy, two of whom had TP53 mutation, and the remaining two had t(3;3) and complex karyotype respectively. Twelve patients (63%) are alive and remain in remission. The median OS for the entire cohort was 11.3 months. Patients who underwent SCT or received DLI had an OS of 12.6 months compared to those who received neither therapy (OS 4.8 months, P = 0.01, hazard ratio (HR) 5.88 (95% confidence interval (CI) 1.164 - 29.7)), which is demonstrated in Figure 1. There was no statistically significant difference in the OS between ELN 2022 favorable/intermediate risk and adverse risk groups (P = 0.44, HR 0.53 (95% CI 0.12 - 2.4)), as shown in Figure 2.
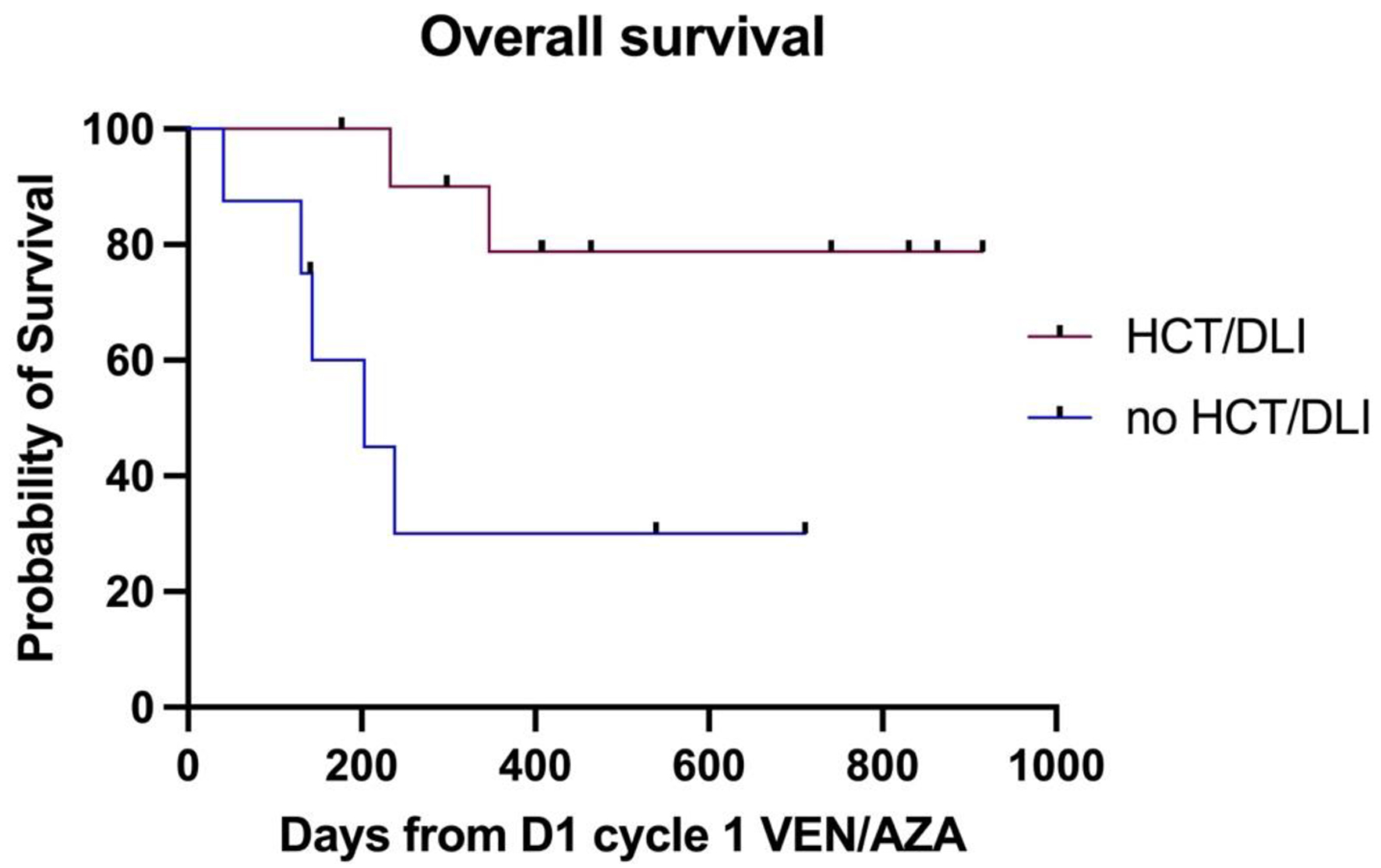 Click for large image | Figure 1. The difference in median OS between patients with and without an allograft or DLI post venetoclax-azacitidine. DLI: donor lymphocyte infusion; OS: overall survival. |
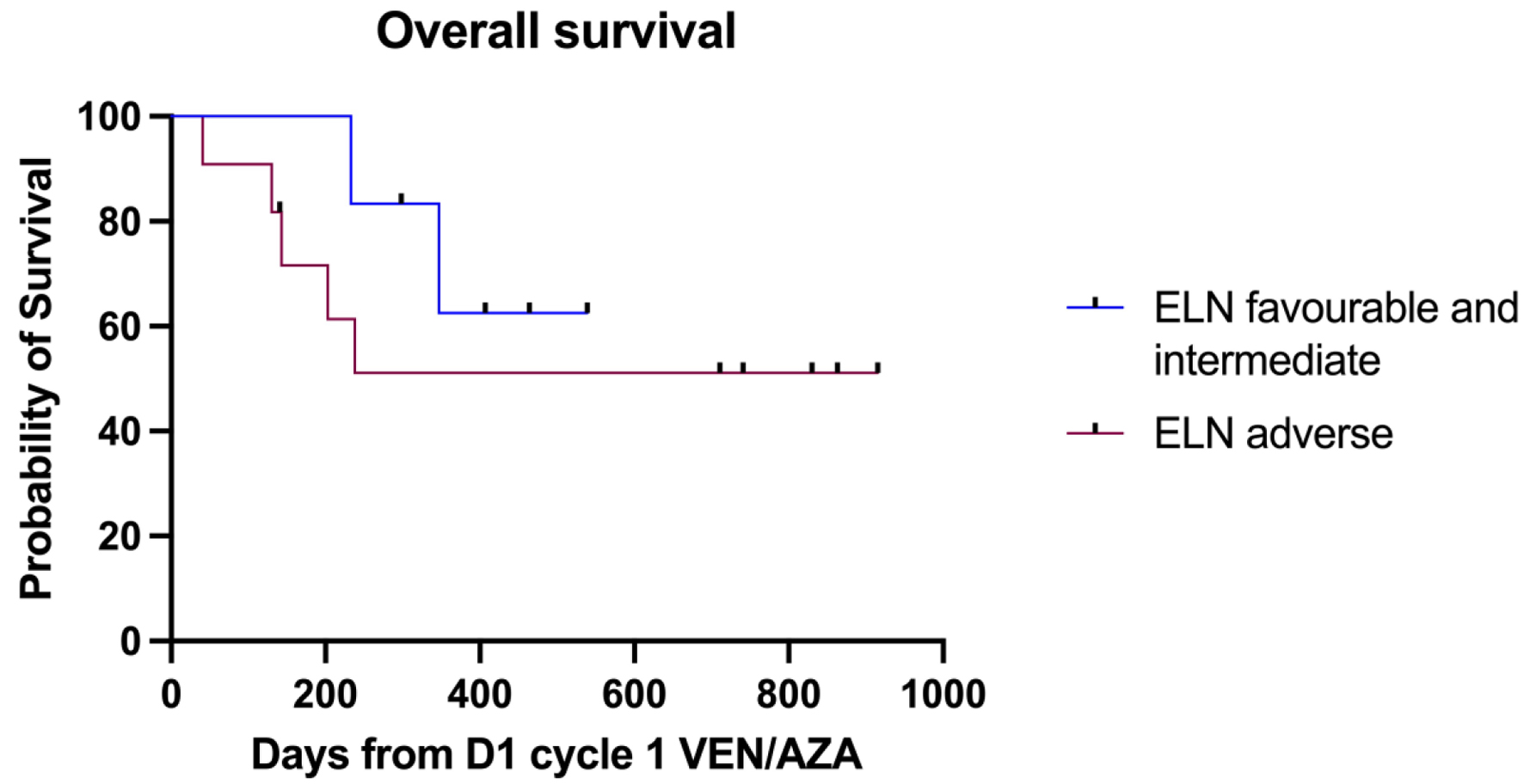 Click for large image | Figure 2. OS as per ELN 2022 risk stratification. ELN: European LeukemiaNet; OS: overall survival. |
Venetoclax-azacitidine is a low-intensity and largely outpatient-based chemotherapy that can be used as a salvage therapy in R/R AML and high-risk MDS. Its relatively low toxicity profile makes it a convenient approach that helps to maintain patient’s fitness, an essential factor for those who receive this treatment combination as a bridge to either SCT or DLI. Furthermore, we were able to demonstrate a survival advantage in patients who had SCT or DLI following remission with venetoclax-azacitidine; however, those results should be cautiously interpreted due to the small cohort size.
Acknowledgments
We wish to thank the hemato-oncology pharmacists, patients and their families.
Financial Disclosure
The authors declare no financial conflict of interest to disclose.
Conflict of Interest
The authors declare no conflict of interest in this study.
Informed Consent
Verbal consent has been obtained from patients and/or their families to obtain and share data for scientific purposes.
Author Contributions
DO analyzed the data and wrote the manuscript. MLM and VM supervised and edited the manuscript. FL generated the survival curve.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70-87.
doi pubmed - American Cancer Society. Cancer Facts & Figures 2024. Atlanta : American Cancer Society; 2024. National Cancer Institute. SEER Cancer Stat Facts: Acute Myeloid Leukemia (AML). 2024. Accessed at https://seer.cancer.gov/statfacts/html/amyl.html on June 3, 2024.
- DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629.
doi pubmed - DeWolf S, Tallman MS. How I treat relapsed or refractory AML. Blood. 2020;136(9):1023-1032.
doi pubmed - Piccini M, Mannelli F, Coltro G. The role of venetoclax in relapsed/refractory acute myeloid leukemia: past, present, and future directions. Bioengineering (Basel). 2023;10(5):591.
doi pubmed - Bazinet A, Garcia-Manero G, Short NJ, Valero YA, Abuasab T, Islam MR, et al. A phase 2 study of the fully oral combination of ASTX727 (decitabine/cedazuridine) plus venetoclax for older and/or unfit patients with acute myeloid leukemia. Blood. 2023;142(Suppl 1):833.
- DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, Kadia TM, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7(10):e724-e736.
doi pubmed - Unglaub JM, Schlenk RF, Middeke JM, Krause SW, Kraus S, Einsele H, Kramer M, et al. Venetoclax-based salvage therapy as bridge-to-transplant is feasible and effective in patients with relapsed/refractory AML. Blood Adv. 2024.
doi pubmed - Kristensen DT, Brondum RF, Orskov AD, Marcher CW, Schollkopf C, Sorensen ALT, Severinsen MT, et al. Venetoclax-based therapy for relapsed or refractory acute myeloid leukaemia following intensive induction chemotherapy. Eur J Haematol. 2023;111(4):573-582.
doi pubmed - Dohner H, DiNardo CD, Appelbaum FR, Craddock C, Dombret H, Ebert BL, Fenaux P, et al. Genetic risk classification for adults with AML receiving less-intensive therapies: the 2024 ELN recommendations. Blood. 2024;144(21):2169-2173.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.