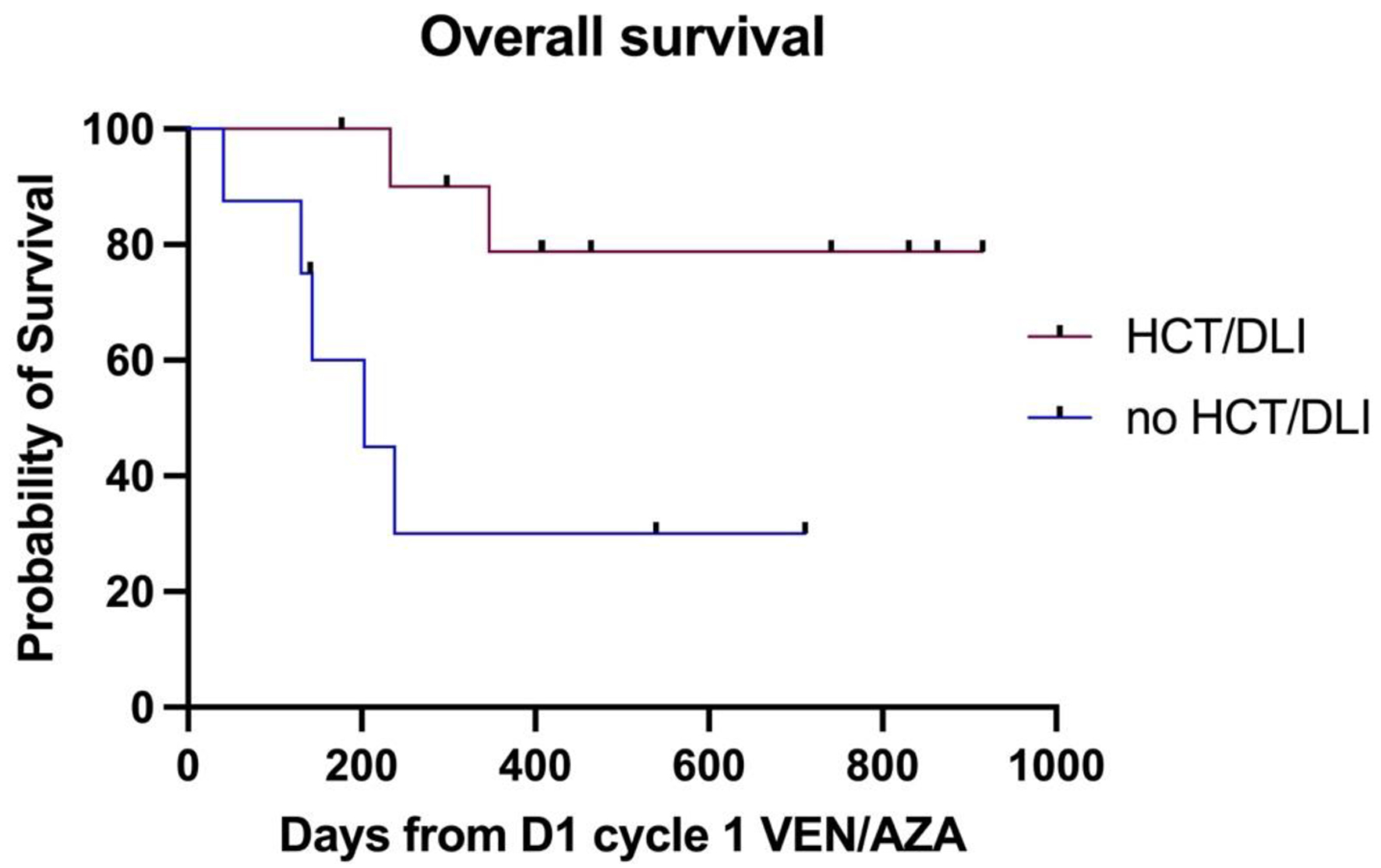
Figure 1. The difference in median OS between patients with and without an allograft or DLI post venetoclax-azacitidine. DLI: donor lymphocyte infusion; OS: overall survival.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Letter to the Editor
Volume 14, Number 2, April 2025, pages 105-108
Venetoclax-Azacitidine Salvage Chemotherapy in Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Single-Center Experience
Figures

Table
| Variable | n (%) |
|---|---|
| AML: acute myeloid leukemia; CR: complete remission; DLI: donor lymphocyte infusion; ELN: European LeukemiaNet; MDS: myelodysplastic syndrome. | |
| Median age | 65 (18 - 77 years) |
| Male/female ratio | 1.1:1 |
| Indication for venetoclax-azacitidine | |
| Primary refractory disease | 6 (31.5%) |
| Relapse post consolidation chemotherapy | 2 (10.5%) |
| Relapse post allogeneic stem cell transplant | 11 (57.8%) |
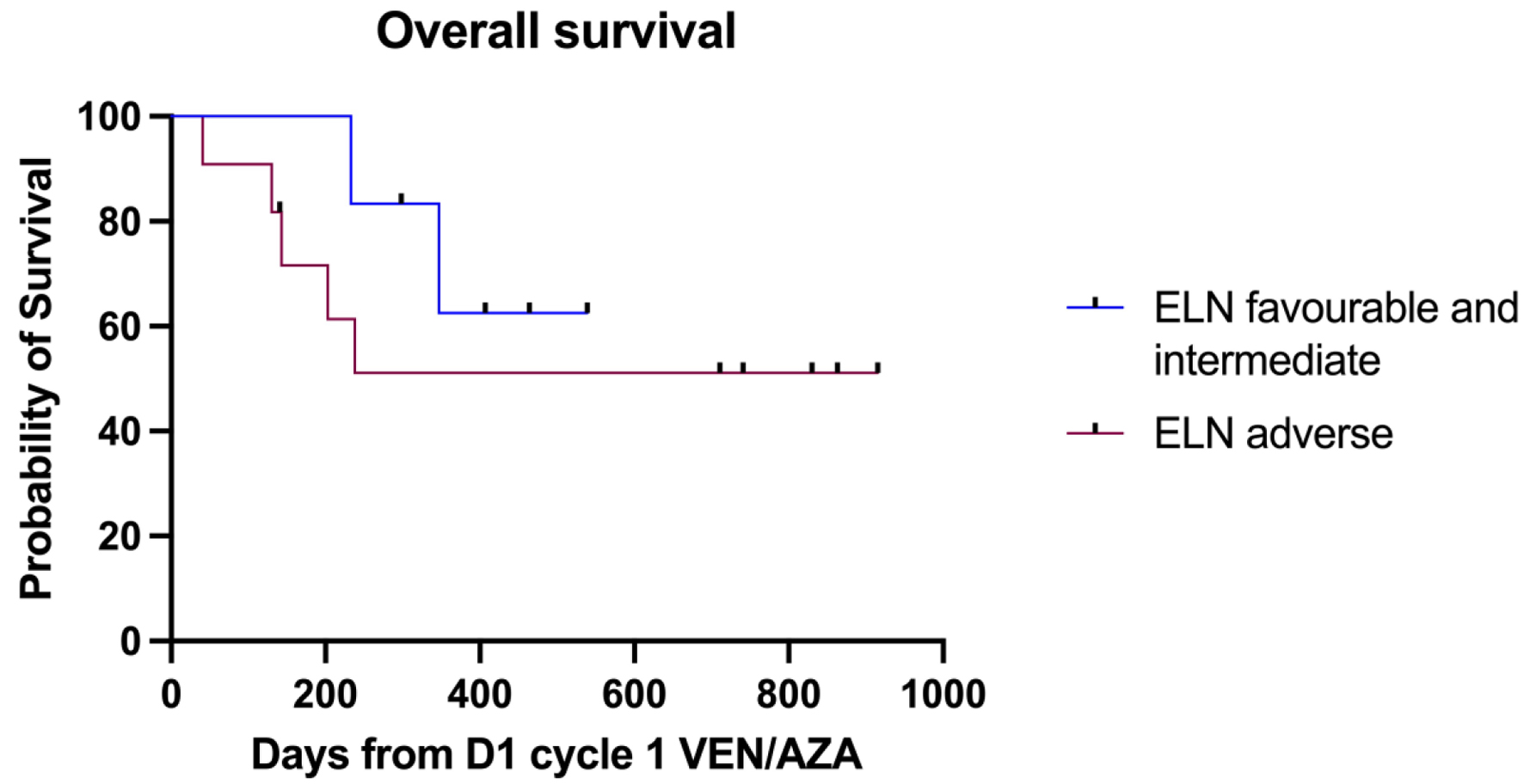
| ELN risk | |
| Favorable risk (NPM1-mutated) | 1 (6.6%) |
| Intermediate risk | 4 (26.6%) |
| Adverse risk | 10 (66.6%) |
| Number of previous lines of AML chemotherapy | |
| 1 line | 12 (80%) |
| 2 lines (including FLAG-Ida) | 3 (20%) |
| Chemotherapy regimen for the MDS patients | |
| Azacitidine | 2 (50%) |
| FLAG-Ida | 1 (25%) |
| Commonest adverse effects | |
| Neutropenia (grade 3-4) | 19 (100%) |
| Admission with neutropenic sepsis | 10 (52.6%) |
| Transfusion requirements | |
| Red cells | 13 (68.4%) |
| Median number of red cell units | 7 (1 - 16) units |
| Platelets | 8 (42.1%) |
| Median number of platelets units | 11 (2 - 23) units |
| Response | |
| CR/CRi | 15 (78.9%) |
| Refractory disease | 4 (20%) |
| Relapse | 1 (5.2%) |
| Consolidation post venetoclax-azacitidine | |
| Allogeneic stem cell transplant | 7 (36%) |
| DLI | 6 (31.5%) |