| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 13, Number 6, December 2024, pages 290-294
Spontaneous Regression of Plasmablastic Lymphoma Associated With Methotrexate After Withdrawal
Takayuki Gotoa, c , Kiichi Hatanob, Nobuhiro Kanemuraa, Hiroki Makitab, Hideko Gotoa
aDepartment of Hematology, Chuno Kosei Hospital, Gifu, Japan
bDepartment of Dentistry and Oral Surgery, Chuno Kosei Hospital, Gifu, Japan
cCorresponding Author: Takayuki Goto, Department of Hematology, Chuno Kosei Hospital, Gifu 501-3802, Japan
Manuscript submitted October 2, 2024, accepted November 13, 2024, published online December 2, 2024
Short title: A Case of PBL Improved With MTX Withdrawal
doi: https://doi.org/10.14740/jh1361
| Abstract | ▴Top |
Plasmablastic lymphoma (PBL) is a malignant lymphoma with poor prognosis that occurs in immunocompromised and elderly patients. We describe the case of a 75-year-old woman with PBL as a methotrexate-associated lymphoproliferative disorder (MTX-LPD). She presented with multiple oral ulcers and mass-like shadows in the lung fields. Biopsy of the oral ulcer revealed medium to large irregular round monotypic B cells positive for cluster of differentiation (CD)138, CD79a, immunoglobulin λ, and Epstein-Barr virus-encoded small ribonucleic acid in situ hybridization, and PBL was diagnosed. The patient showed negative results for human immunodeficiency virus and had a history of taking MTX for rheumatoid arthritis, suggesting MTX-LPD. Following discontinuation of MTX, the oral ulcers resolved 1 month later without recurrence, and lung lesions decreased in size over time. Because MTX-LPD can take the form of PBL and may resolve with MTX withdrawal alone, therapeutic interventions should be carefully considered. While PBL is typically highly aggressive and requires prompt treatment, MTX-LPD cases can sometimes resolve without further treatment, depending on the clinical course. However, in cases where the disease shows progression or when spontaneous regression does not occur, additional therapeutic interventions may be necessary to manage the disease effectively.
Keywords: Plasmablastic lymphoma; Methotrexate; Lymphoproliferative disorder
| Introduction | ▴Top |
Plasmablastic lymphoma (PBL) is a rare subtype of B-cell non-Hodgkin lymphoma [1]. According to the 2017 World Health Organization classification, this pathology is categorized as a lymphoid neoplasm with plasmablastic differentiation, representing 2% of lymphomas related to acquired immunodeficiency syndrome [2]. Because of its rarity, no standard of care has been established for PBL, and outcomes remain poor [3]. PBL commonly occurs in immunocompromised patients with human immunodeficiency virus (HIV) infection and in the elderly, and HIV-negative PBL may develop as a type of lymphoproliferative disorder (LPD) [1]. We report a case of PBL that developed as a methotrexate (MTX)-associated LPD (MTX-LPD) and resolved spontaneously after MTX withdrawal alone.
| Case Report | ▴Top |
A 75-year-old woman presented with intractable stomatitis and multiple oral ulcers. She had been diagnosed with rheumatoid arthritis 10 years earlier and was taking MTX at a dose of 10 mg/week as treatment. Multiple perforating ulcer lesions were found in the oral cavity. Laboratory testing revealed lymphocytosis (6,688/µL), elevated levels of soluble interleukin (IL)-2 receptor (4,743 U/mL) and anemia (hemoglobin: 101 g/L). Both lactate dehydrogenase levels (214 U/L; reference range: 124 - 222 U/L) and serum creatinine levels (0.71 mg/dL) were within the normal range. The serum calcium level was 7.9 mg/dL; however, due to hypoalbuminemia, the corrected calcium level was 10.2 mg/dL, which was within the normal range. Serum protein electrophoresis revealed no M-protein.
Biopsy of the oral ulcer revealed infiltration of medium to large irregular round monotypic B cells with a high nuclear-to-cytoplasmic ratio, with partial expression of cluster of differentiation (CD)138, CD20, CD79a, immunoglobulin (Ig) λ, and Epstein-Barr virus (EBV)-encoded small ribonucleic acid (RNA) (EBER) in situ hybridization, and negative results for CD3, CD5, and CD10 with immunohistochemical staining (Fig. 1). The diagnosis of PBL was thus confirmed. Serological examination showed negative results for HIV antigen and antibody. For EBV infection, anti-viral capsid antigen (VCA) IgG and anti-Epstein-Barr nuclear antigen (EBNA) antibodies were positive, while anti-VCA IgM and anti-early antigen diffuse-related (EA-DR) IgG were negative. EBV deoxyribonucleic acid (DNA) quantitative polymerase chain reaction (qPCR) assay showed 3.52 log10 copies/mL. Since the patient had a history of MTX treatment, PBL was diagnosed as a type of MTX-LPD, and MTX was immediately discontinued.
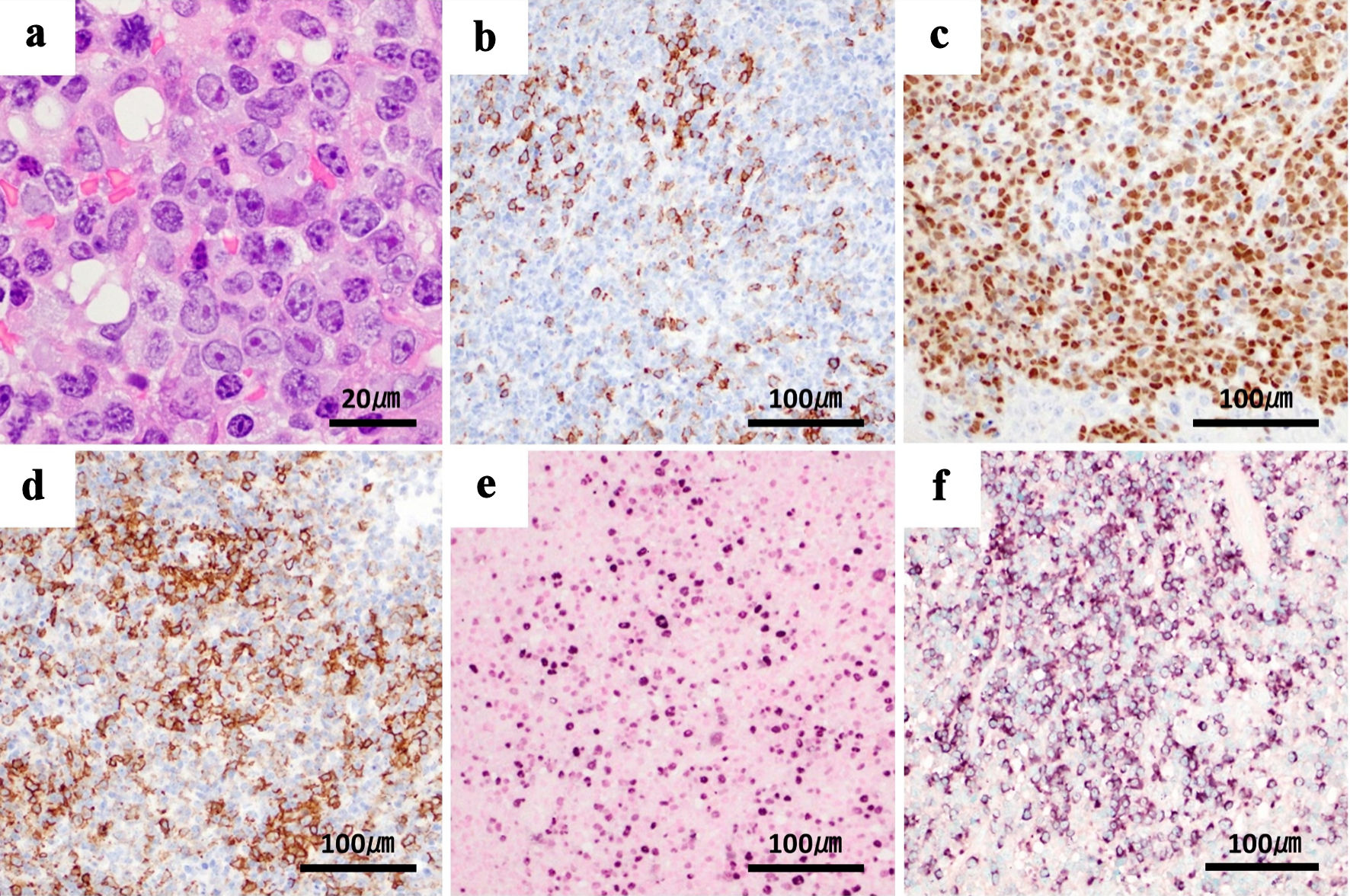 Click for large image | Figure 1. Biopsy of the oral ulcer shows tumor cells with plasmablastic differentiation. Tumor cells show immunoreactivity for plasma cell-associated antigen MUM-1 and partial reactivity for CD138, variable positivity for B-cell markers CD20 and CD79a, and cytoplasmic λ light chain restriction. EBV is present, and MIB-1 is positive in over 90% of cells. These findings are compatible with plasmablastic lymphoma. (a) Tumor cells in oral ulcer (hematoxylin and eosin staining), × 100. (b) Immunoblastic cells exhibiting immunoreactivity with CD138. (c) MUM-1. (d) CD20. (e) Nuclear positivity with EBER in situ hybridization. (f) Cytoplasmic λ light chain restriction, × 20. MUM-1: multiple myeloma oncogene 1; CD: cluster of differentiation; EBV: Epstein-Barr virus; EBER: EBV-encoded small RNA; RNA: ribonucleic acid; MIB-1: monoclonal antibody Ki-67. |
Positron emission tomography (PET)-computed tomography (CT) revealed 18F-fluorodeoxyglucose (FDG) accumulation in the right mandibular ulcer. Multiple nodular lesions were observed in the lung fields but showed no accumulation of FDG on imaging studies (Fig. 2). Additionally, there was no FDG accumulation in the bone or bone marrow. Transbronchial needle aspiration was performed to diagnose the pulmonary multiple masses, but the specimen obtained proved insufficient. The patient then underwent transesophageal endoscopic ultrasound-guided fine-needle aspiration, but no diagnosis was established, because only necrotic tissue was obtained; this tissue was assumed to represent tumor necrosis. No bacteria or fungi were detected in the specimen. After discontinuation of MTX, oral ulcer lesions gradually improved and scarring occurred. Chest CT revealed reductions in the size of multiple pulmonary nodular lesions at 2 weeks and 1 month after MTX withdrawal (Fig. 3). The patient was therefore carefully monitored without additional antineoplastic agents. CT of the chest at 3 and 6 months after diagnosis showed no worsening of nodular lesions, and blood tests at 3 months after diagnosis showed decreases in both soluble IL-2 receptor (955 U/mL) and EBV DNA qPCR assay (< 2.18 log10 copies/mL). The lymphocyte counts gradually decreased at 1 month (2,643/µL) and at 6 months (1,103/µL) after diagnosis. Follow-up evaluations, including EBV DNA qPCR assay, chest CT and assessments of the oral ulcer lesion, have been conducted every 3 months over the past year, with no signs of recurrence observed. As of the time of writing, the patient remains free from recurrence.
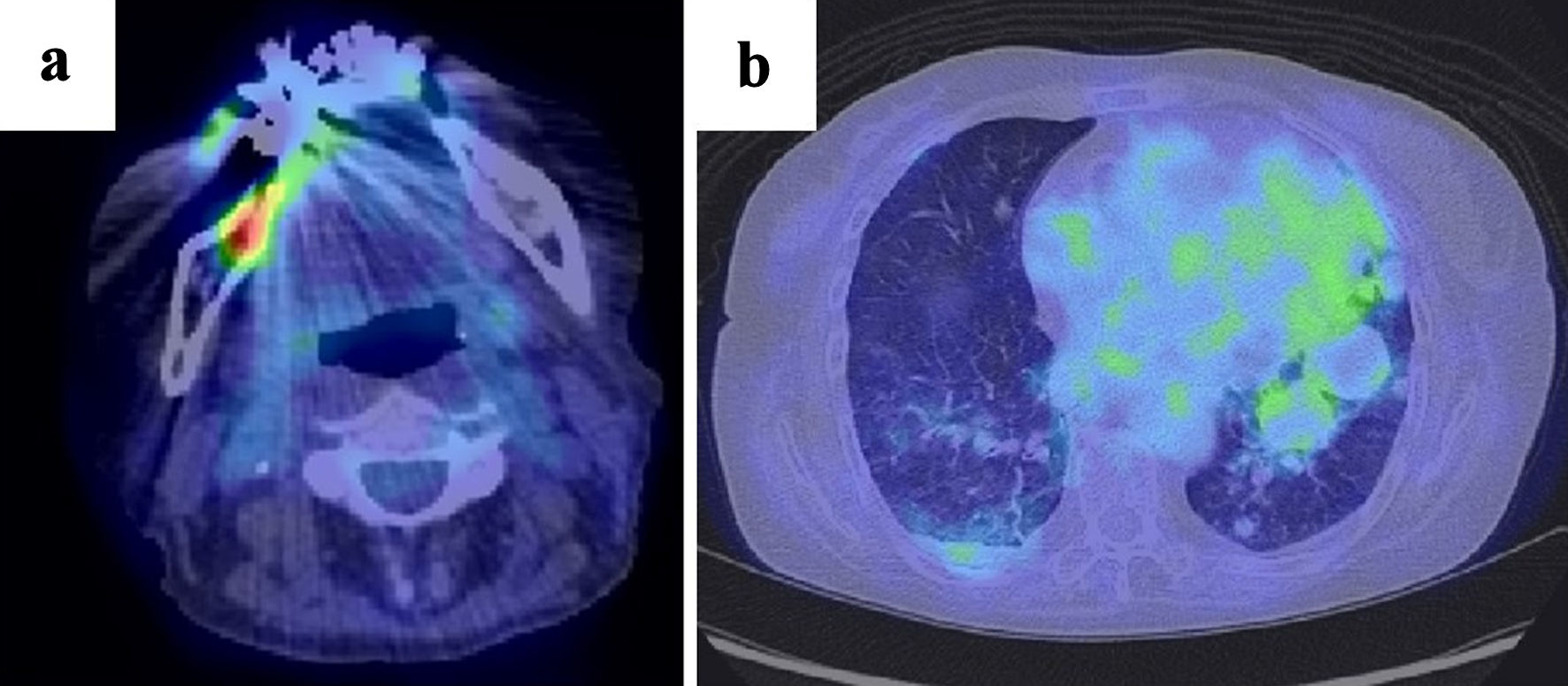 Click for large image | Figure 2. (a) PET-CT shows FDG accumulation in the known right mandibular tumor. (b) Multiple mass-like shadows are observed in the lung fields, but no FDG accumulation is noted. PET-CT: positron emission tomography-computed tomography; FDG: 18F-fluorodeoxyglucose. |
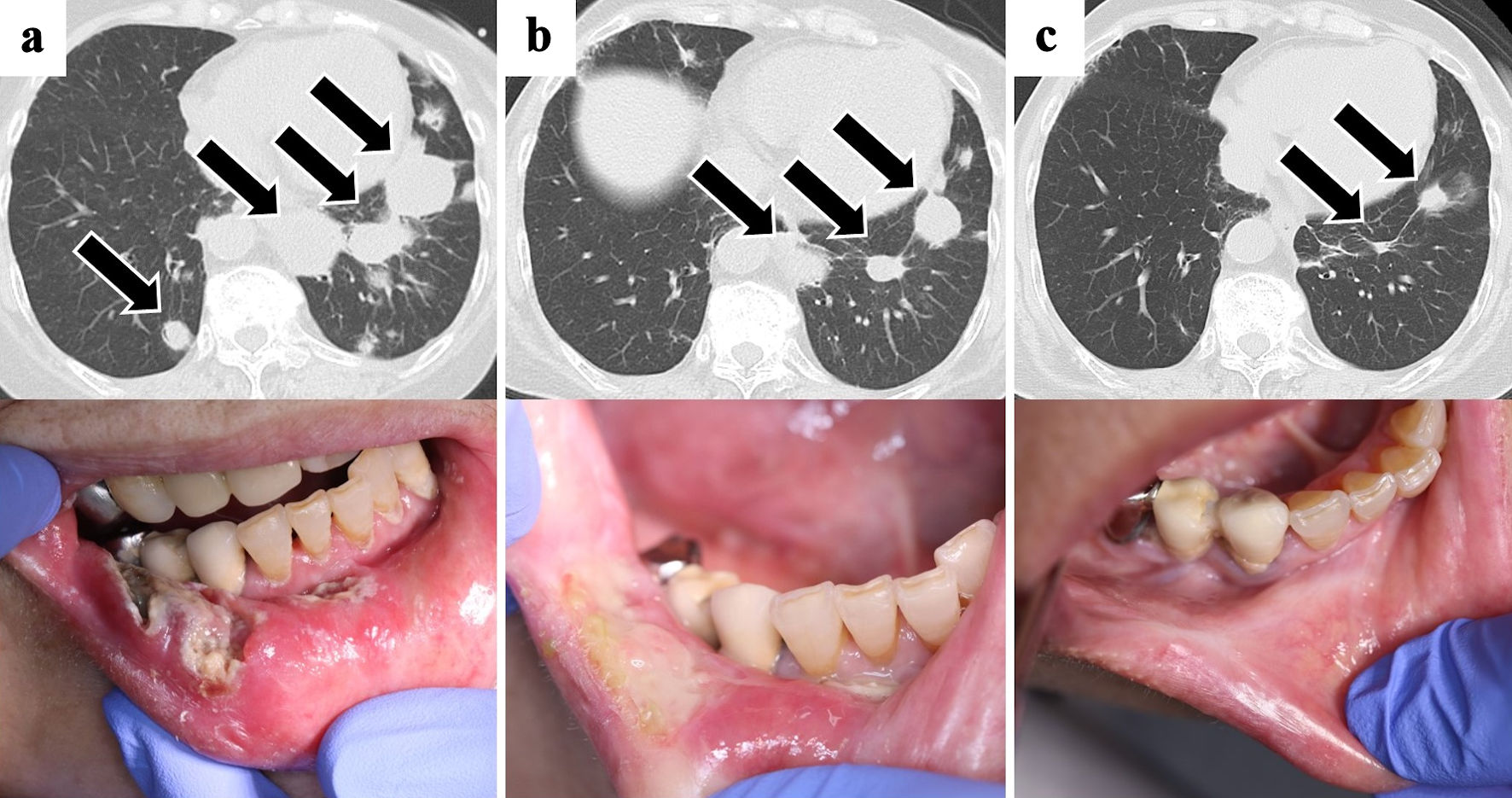 Click for large image | Figure 3. Chest CT and oral ulcer findings. The oral ulcer lesions and multiple pulmonary nodules (arrows) reduced with only discontinuation of methotrexate. (a) At the time of initial diagnosis. (b) One month later. (c) Three months later. CT: computed tomography. |
| Discussion | ▴Top |
Here, we have described a case of HIV-negative, EBV-positive PBL. Based on a retrospective cohort of 590 individual cases of PBL, Castillo et al [1] reported that HIV-negative PBL represented a minority of cases (28%) and was more common in female and older patients compared to HIV-positive PBL. The most common presentation for both HIV-positive and HIV-negative PBL is the oral cavity and/or jaw, occurring in 48% and 40% of patients, respectively [1]. A retrospective study by Li et al [4] found that among 309 HIV-negative PBL patients, 56% were EBER-positive [1, 4].
PBL is a high-grade neoplasm with cytomorphological features such as large immunoblasts or large plasma cells that express plasma cell markers, and either lack or have only dim expression of B-cell markers [5]. The PBL immunophenotype involves positivity for multiple myeloma oncogene 1 (MUM-1), B lymphocyte-induced maturation protein 1 (BLIMP-1), CD38, and CD138 [6], and weak expression of CD20, CD79a, and paired box 5 (PAX-5), often with Ig chain restriction [7]. Plasmablastic or anaplastic multiple myeloma may be morphologically and immunophenotypically identical to PBL, and these entities are sometimes impossible to distinguish [1]. In this case, findings in support of PBL included an immunophenotype with expression of MUM-1, CD138, and EBER, and light chain restriction. Additionally, given the patient’s history of MTX use and spontaneous regression after MTX discontinuation, an MTX-LPD pathology was also considered. On the other hand, no features favored myeloma, including the presence of monoclonal paraproteinemia, hypercalcemia, renal dysfunction, or lytic bone lesions.
The prognosis of PBL is very poor even with administration of cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone, or more intensive treatments [1]. In an international study of 281 patients with PBL, median overall survival (OS) was 2.76 years (95% confidence interval (CI): 1.7 - 4.25 years) and an estimated 5-year OS of 36% (95% CI: 30 - 42%). EBV-lymphoma, poor performance status, advanced stage, and bone marrow involvement were associated with poorer OS. Immunodeficiency was identified in 51% of PBL, including 35% with HIV [8].
MTX-LPD is an iatrogenic immunodeficiency-associated LPD in which lymphoma or lymphoproliferation occurs in an immunocompromised patient in association with MTX administration, often in the context of EBV infection [9]. The histopathology of MTX-LPD is diverse, with diffuse large B-cell lymphoma accounting for half of cases, and Hodgkin lymphoma accounting for about 10% [10]; however, no reports are available on the incidence of PBL associated with MTX-LPD. Although most cases of HIV-negative PBL show no immune abnormalities, a small number of cases arising from lymphoproliferative or autoimmune diseases have been reported [11]. In this case, PBL was diagnosed based on a biopsy sample from an intraoral lesion. The pathology was considered a type of MTX-LPD because the patient was negative for HIV and had a history of treatment with MTX. Prompt initiation of combination chemotherapy was considered because of the presence of concomitant pulmonary lesions and the generally rapid progression of PBL. However, both the oral and pulmonary lesions improved with discontinuation of MTX alone, and the decline in EBV DNA levels supported our decision to consider chemotherapy unnecessary. A small number of cases of PBL due to MTX treatment have been reported, with clinical courses similar to that of general PBL and early death after diagnosis [12].
Saito et al [13] reported that about one-third of patients with MTX-LPD who discontinued MTX required further treatment. Of those who received treatment, one-quarter experienced relapse. The 5-year survival rate for patients who achieved complete response (CR) post-treatment was 67.2%, while those who relapsed had a 5-year survival rate of 72.2% [13]. Ichikawa et al [14] studied 47 cases where MTX was discontinued. Among these, 19 patients experienced disease progression, and three out of five patients who initially showed spontaneous regression but later relapsed underwent chemotherapy, with approximately 80% receiving R-CHOP or R-THP-COP regimens. CR was achieved in 18 patients [14]. According to these reports, approximately 60% of MTX-LPD cases have shown spontaneous regression after discontinuation of MTX [13, 14]. Cessation of the drug and confirmation of response is thus a valid treatment option.
Similar to the present case, we identified reports of two patients with rheumatoid arthritis in whom PBL regressed spontaneously with only reduction or withdrawal of MTX [15, 16]. It has been reported that in cases of MTX-LPD showing spontaneous regression, lymphocyte counts decrease after starting MTX and recover after discontinuation of the drug [17]. Furthermore, cases with a lymphocyte count of 1,000/µL or more at diagnosis and 6 months post-MTX withdrawal have been associated with better treatment-free survival [18]. In our case, while a recovery in lymphocyte count post-MTX withdrawal was not observed and instead showed a downward trend, the high initial lymphocyte count and maintenance of over 1,000/µL at 6 months may have contributed to the sustained spontaneous regression of PBL. The mechanisms and characteristics of spontaneous regression remain unclear. Anti-tumor immunity is presumably restored, and tumor necrosis occurs after MTX withdrawal [19], and spontaneous regression is reportedly significantly more common in EBV-positive LPD than in EBV-negative LPD [14].
Conclusions
We encountered a case of PBL that developed as MTX-LPD and spontaneously regressed with only discontinuation of MTX. In general, PBL is a high-grade disease with an aggressive course and poor prognosis, but in the case of MTX-LPD, spontaneous resolution may occur with only MTX withdrawal, and the timing of therapeutic intervention should be carefully considered. The mechanisms and characteristics of cases that resolve spontaneously are still unknown, and treatment for MTX-LPD remains to be established. Validation of the present findings is a subject for future research.
Acknowledgments
I would like to thank Dr. Tsuyoshi Takami from the Tohkai Cytopathology Institute for providing the histopathological images.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Consent for publication of this case report was obtained from the patient.
Author Contributions
TG contributed to patient care, the clinical data analysis, and writing of the manuscript. NK contributed to the interpretation of the results, and writing of the manuscript. HG contributed to the patient care, the clinical data analysis, and critical review of the manuscript. KH and HM contributed to patient diagnosis, follow-up, the clinical data analysis and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PBL: plasmablastic lymphoma; HIV: human immunodeficiency virus; LPD: lymphoproliferative disorder; MTX: methotrexate; IL: interleukin; CD: cluster of differentiation; Ig: immunoglobulin; EBV: Epstein-Barr virus; RNA: ribonucleic acid; EBER: Epstein-Barr virus-encoded small RNA; MTX-LPD: methotrexate-associated lymphoproliferative disorder; VCA: viral capsid antigen; EBNA: Epstein-Barr nuclear antigen; EA-DR: early antigen diffuse-related; DNA: deoxyribonucleic acid; qPCR: quantitative polymerase chain reaction; PET: positron emission tomography; CT: computed tomography; FDG: 18F-fluorodeoxyglucose; MUM-1: multiple myeloma oncogene 1; BLIMP-1: B lymphocyte-induced maturation protein 1; PAX-5: paired box 5; OS: overall survival; CI: confidence interval; CR: complete response
| References | ▴Top |
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125(15):2323-2330.
doi pubmed - Kaur S, Kollimuttathuillam S. Plasmablastic lymphoma: past, present, and future. Clin Lymphoma Myeloma Leuk. 2023;23(9):e253-e259.
doi pubmed - Bibas M, Castillo JJ. Current knowledge on HIV-associated plasmablastic lymphoma. Mediterr J Hematol Infect Dis. 2014;6(1):e2014064.
doi pubmed - Li YJ, Li JW, Chen KL, Li J, Zhong MZ, Liu XL, Yi PY, et al. HIV-negative plasmablastic lymphoma: report of 8 cases and a comprehensive review of 394 published cases. Blood Res. 2020;55(1):49-56.
doi pubmed - Bailly J, Jenkins N, Chetty D, Mohamed Z, Verburgh ER, Opie JJ. Plasmablastic lymphoma: an update. Int J Lab Hematol. 2022;44(Suppl 1):54-63.
doi pubmed - Vega F, Chang CC, Medeiros LJ, Udden MM, Cho-Vega JH, Lau CC, Finch CJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles. Mod Pathol. 2005;18(6):806-815.
doi pubmed - Coppo P, Ricard L. Plasmablastic lymphoma: better refine prognosis. Blood. 2024;143(2):101-102.
doi pubmed - Di Ciaccio PR, Polizzotto MN, Cwynarski K, Gerrie AS, Burton C, Bower M, Kuruvilla J, et al. The influence of immunodeficiency, disease features, and patient characteristics on survival in plasmablastic lymphoma. Blood. 2024;143(2):152-165.
doi pubmed - Momose S, Tamaru JI. Iatrogenic immunodeficiency-associated lymphoproliferative disorders of B-cell type that develop in patients receiving immunosuppressive drugs other than in the post-transplant setting. J Clin Exp Hematop. 2019;59(2):48-55.
doi pubmed - Kurita D, Miyoshi H, Ichikawa A, Kato K, Imaizumi Y, Seki R, Sato K, et al. Methotrexate-associated Lymphoproliferative Disorders in Patients With Rheumatoid Arthritis: Clinicopathologic Features and Prognostic Factors. Am J Surg Pathol. 2019;43(7):869-884.
doi pubmed - Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, Colvin GA, et al. HIV-negative plasmablastic lymphoma: not in the mouth. Clin Lymphoma Myeloma Leuk. 2011;11(2):185-189.
doi pubmed - Tabata R, Tabata C, Uesugi H, Takei Y. Highly aggressive plasmablastic neoplasms in patients with rheumatoid arthritis treated with methotrexate. Int Immunopharmacol. 2019;68:213-217.
doi pubmed - Saito R, Tanaka M, Ito H, Kuramoto N, Fujii T, Saito S, Kaneko Y, et al. Overall survival and post-spontaneous regression relapse-free survival of patients with lymphoproliferative disorders associated with rheumatoid arthritis: a multi-center retrospective cohort study. Mod Rheumatol. 2022;32(1):50-58.
doi pubmed - Ichikawa A, Arakawa F, Kiyasu J, Sato K, Miyoshi H, Niino D, Kimura Y, et al. Methotrexate/iatrogenic lymphoproliferative disorders in rheumatoid arthritis: histology, Epstein-Barr virus, and clonality are important predictors of disease progression and regression. Eur J Haematol. 2013;91(1):20-28.
doi pubmed - Garcia-Noblejas A, Velasco A, Cannata-Ortiz J, Arranz R. [Spontaneous regression of immunodeficiency associated plasmablastic lymphoma related to methotrexate after decrease of dosage]. Med Clin (Barc). 2013;140(12):569-570.
doi pubmed - Yordanova K, Stilgenbauer S, Bohle RM, Lesan V, Thurner L, Kaddu-Mulindwa D, Bittenbring JT, et al. Spontaneous regression of a plasmablastic lymphoma with MYC rearrangement. Br J Haematol. 2019;186(6):e203-e207.
doi pubmed - Tokuhira M, Tanaka Y, Takahashi Y, Kimura Y, Tomikawa T, Anan T, Watanabe J, et al. The clinical impact of absolute lymphocyte count in peripheral blood among patients with methotrexate - associated lymphoproliferative disorders. J Clin Exp Hematop. 2020;60(2):41-50.
doi pubmed - Saito S, Kaneko Y, Yamaoka K, Tokuhira M, Takeuchi T. Distinct patterns of lymphocyte count transition in lymphoproliferative disorder in patients with rheumatoid arthritis treated with methotrexate. Rheumatology (Oxford). 2017;56(6):940-946.
doi pubmed - Omameuda T, Miyato H, Sata N, Lefor AK. Primary hepatic methotrexate-associated lymphoproliferative disorder associated with Epstein-Barr virus reactivation and accompanied by spontaneous necrosis: A case report. Medicine (Baltimore). 2022;101(47):e31993.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.