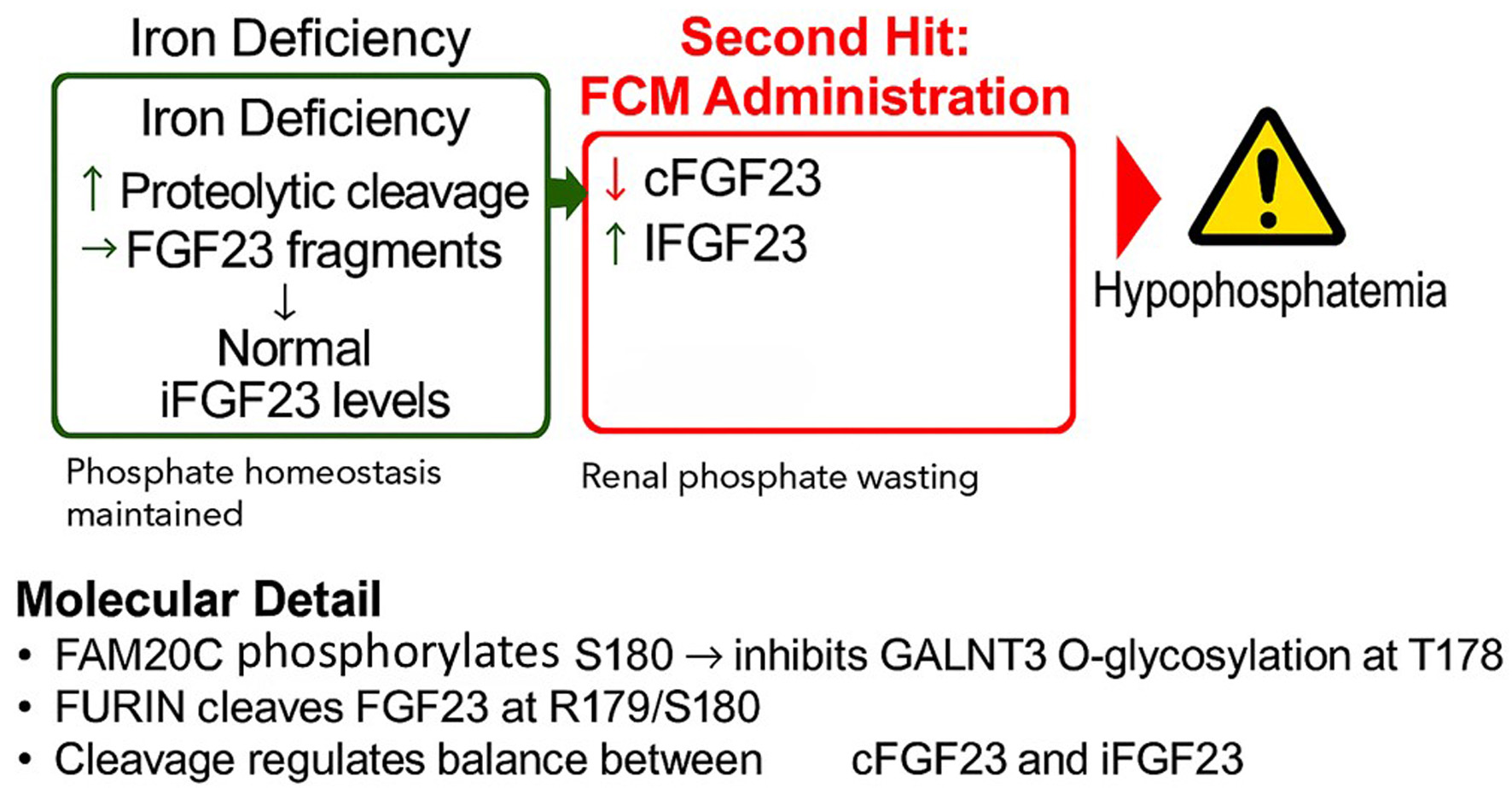
Figure 1. The proposed “two-hit” mechanism of ferric carboxymaltose (FCM)-induced hypophosphatemia. (a) In a state of iron deficiency, transcription of the FGF23 gene is upregulated in osteocytes, leading to increased protein production. However, this is matched by a compensatory increase in proteolytic cleavage, resulting in high levels of inactive C-terminal FGF23 (cFGF23) fragments but normal levels of active, intact FGF23 (iFGF23). Phosphate homeostasis is maintained. (b) The administration of FCM is hypothesized to inhibit the cleavage process. This uncouples the high production rate from degradation, leading to a surge in circulating iFGF23, which in turn causes renal phosphate wasting and hypophosphatemia. (c) Molecular detail of the FGF23 cleavage site. The protease FURIN cleaves the protein at R179/S180. This cleavage is promoted by the kinase FAM20C, which phosphorylates serine 180 (S180). This phosphorylation prevents the protective O-glycosylation of threonine 178 (T178) by the enzyme GALNT3.