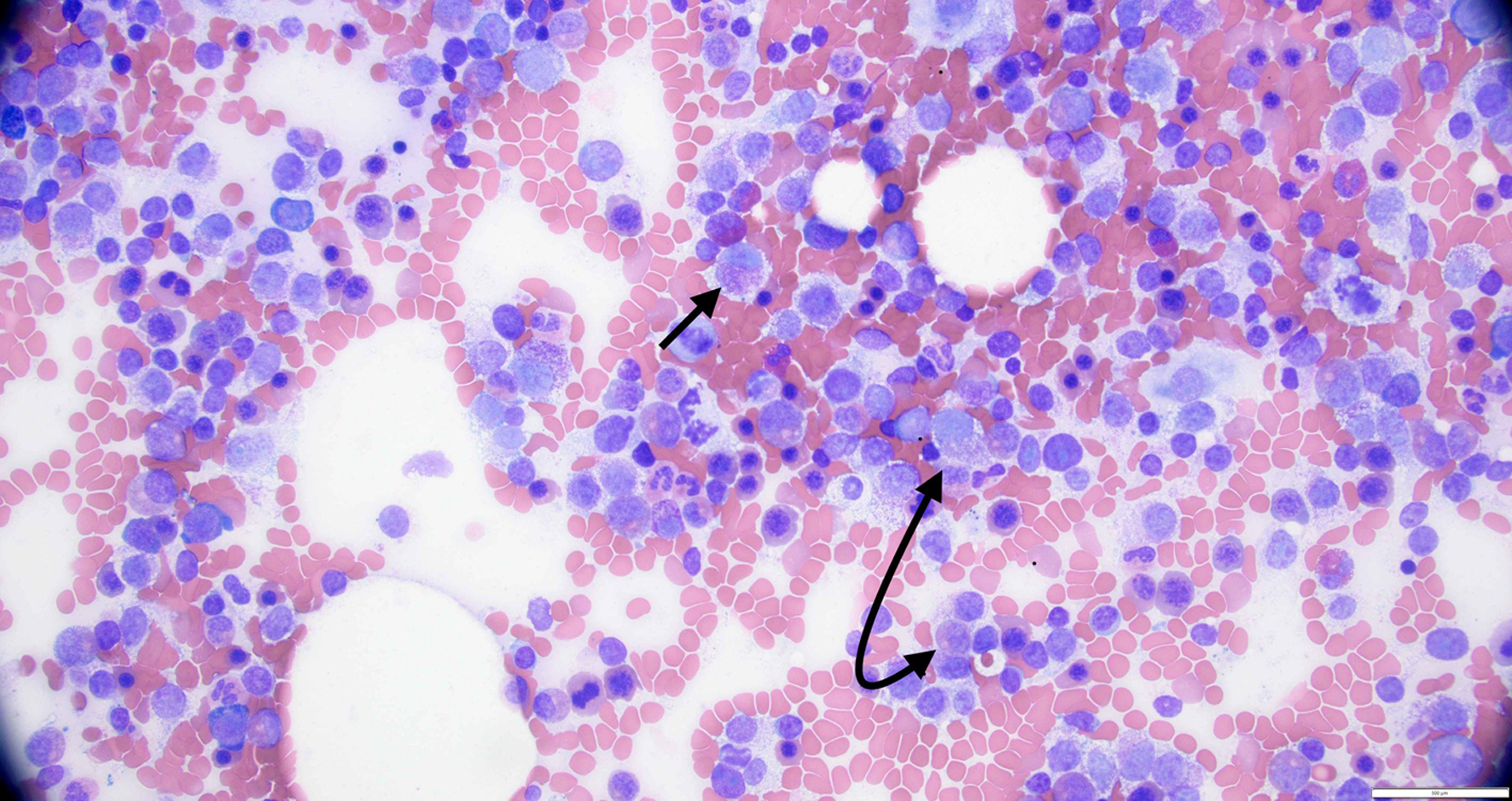
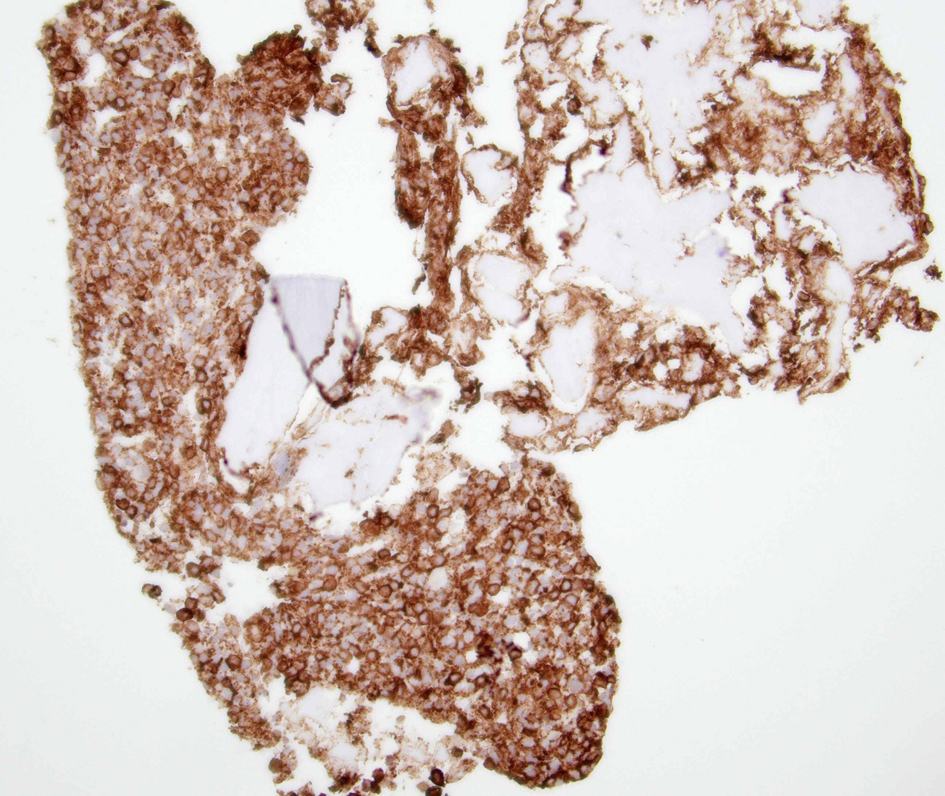
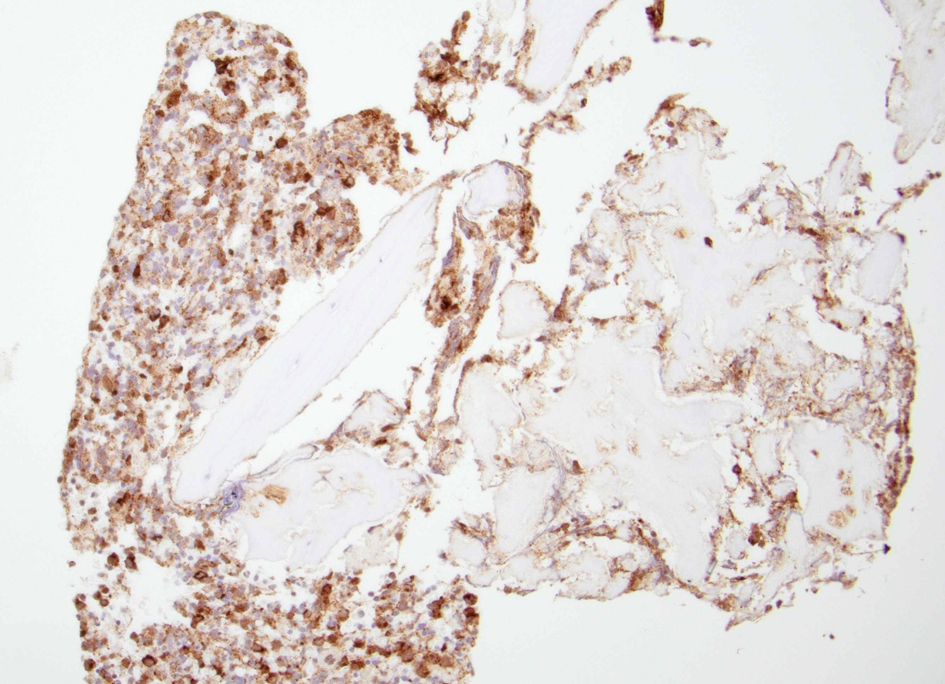
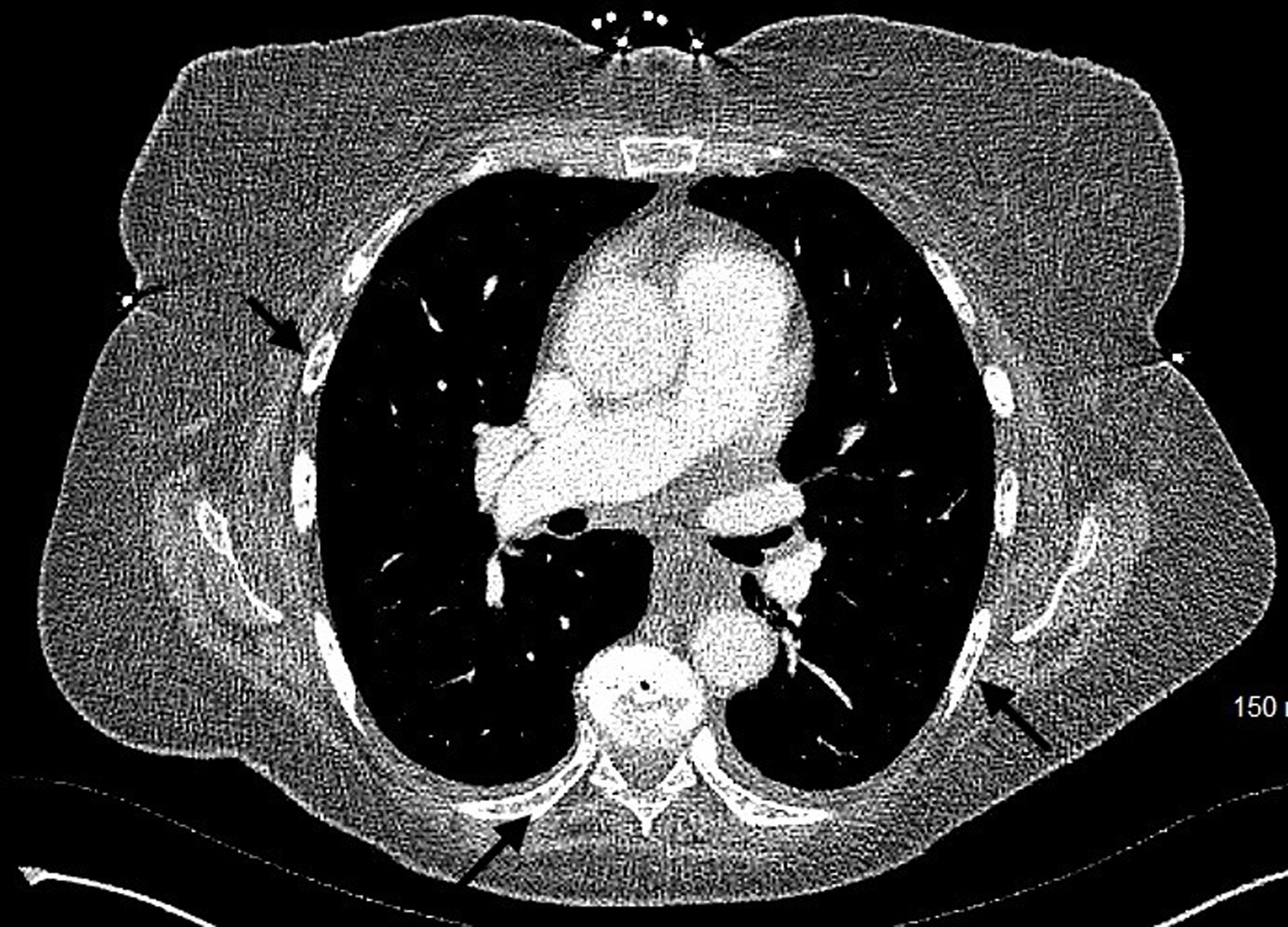
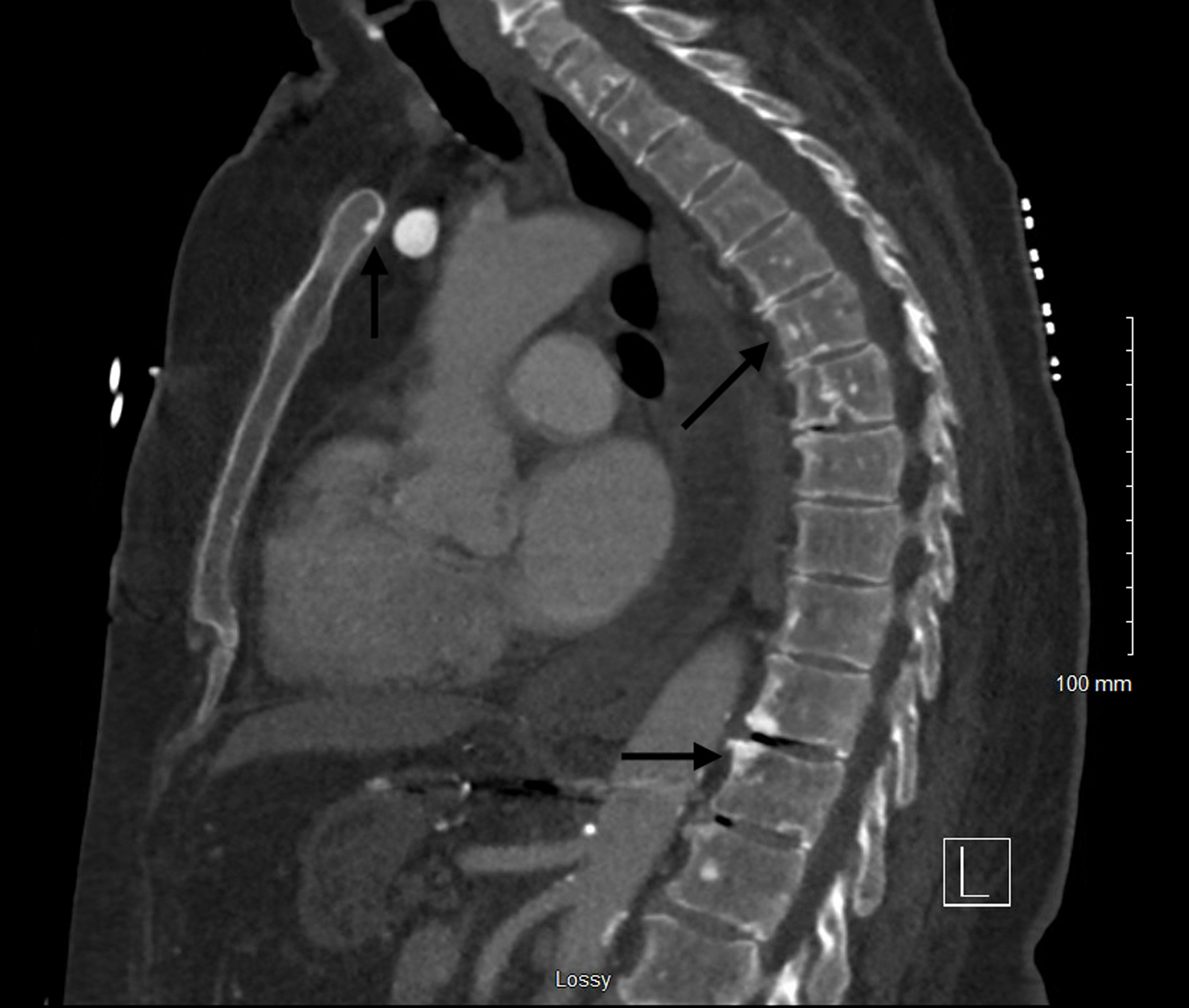
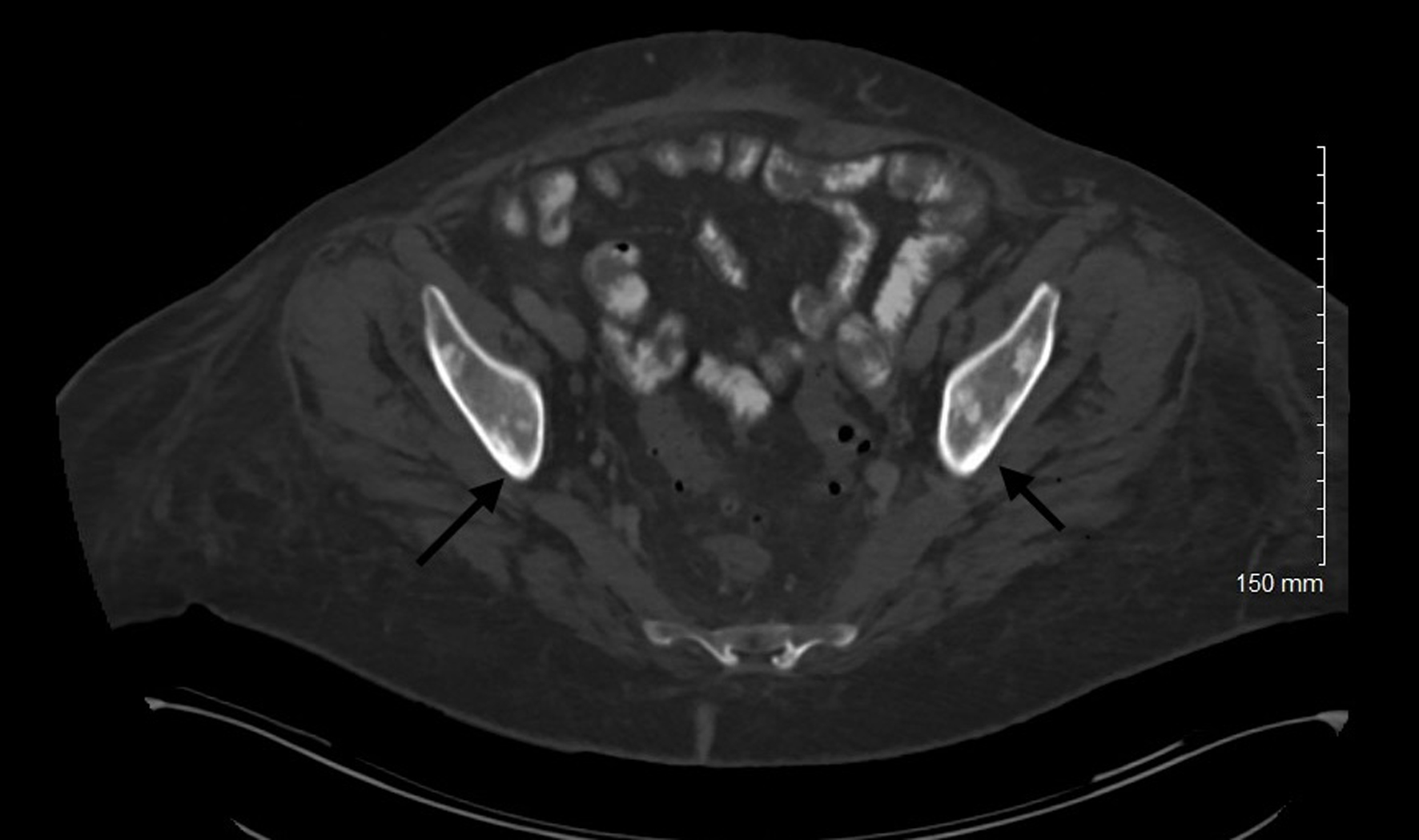
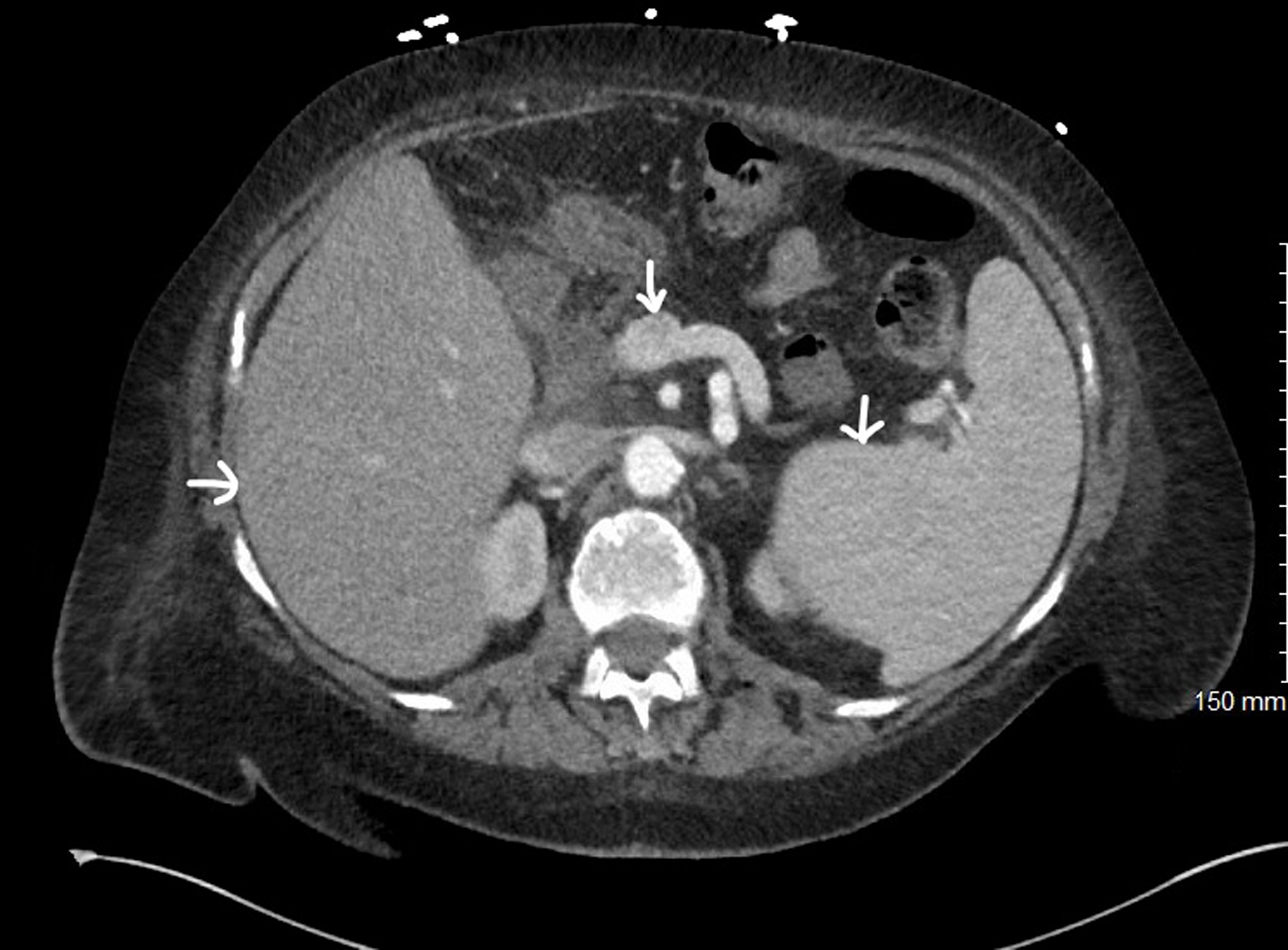
Figure 1. High power view of bone marrow biopsy showing > 20% atypical mast cells (black arrows).
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Review
Volume 14, Number 4, August 2025, pages 174-192
Mast Cell Leukemia: Comprehensive Review of Literature With Current Insights and Updates on Management
Figures

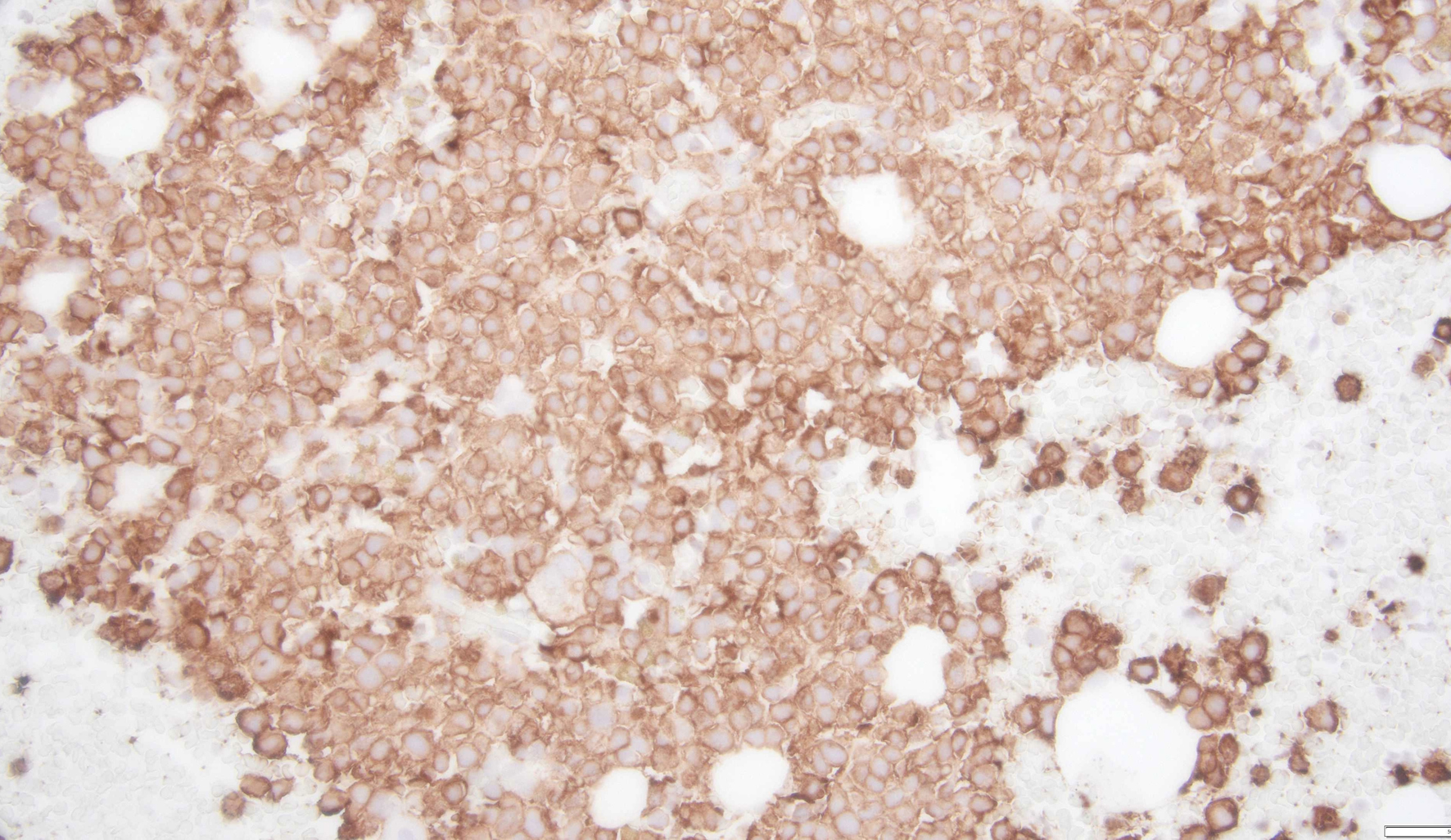
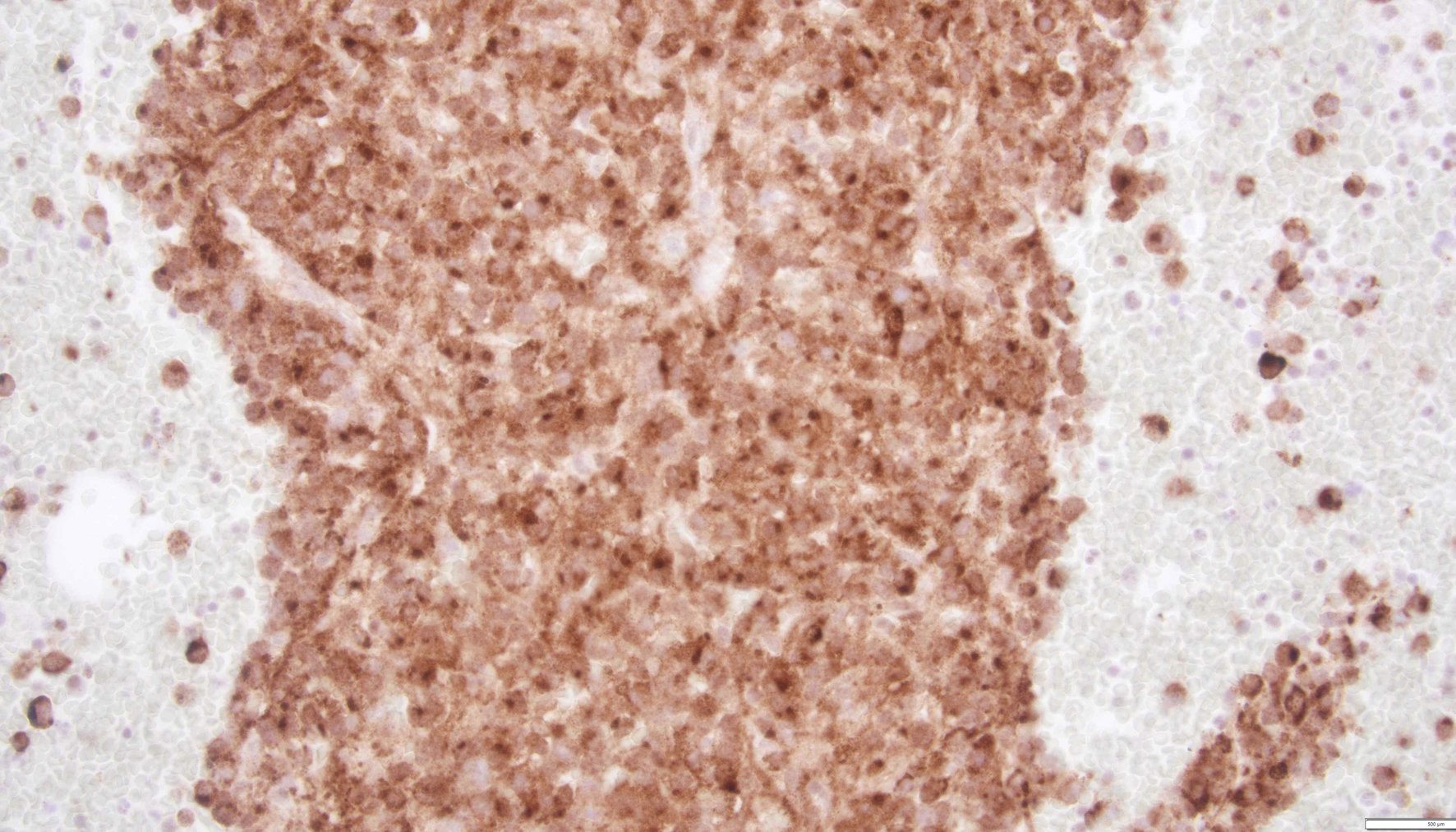
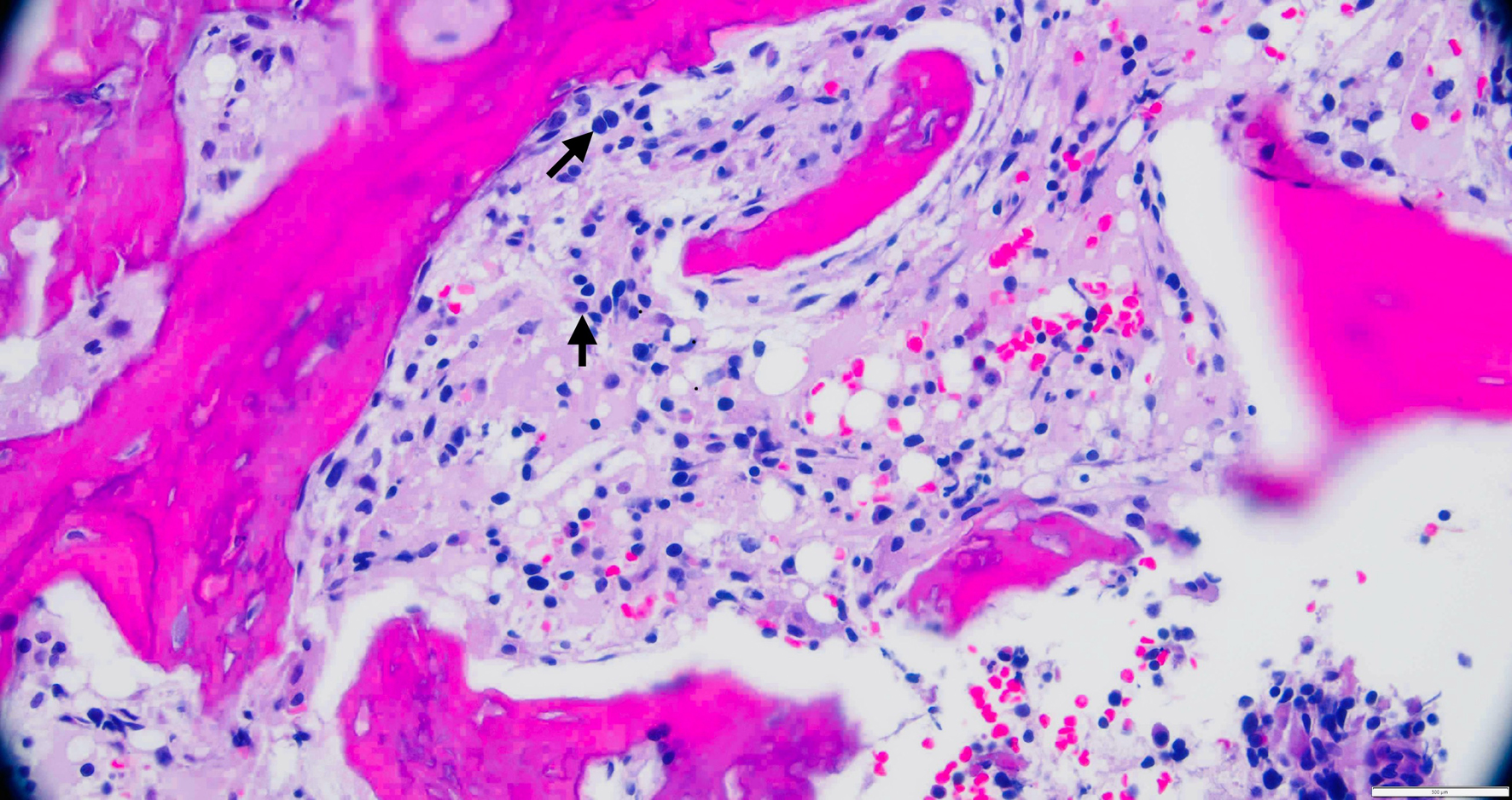
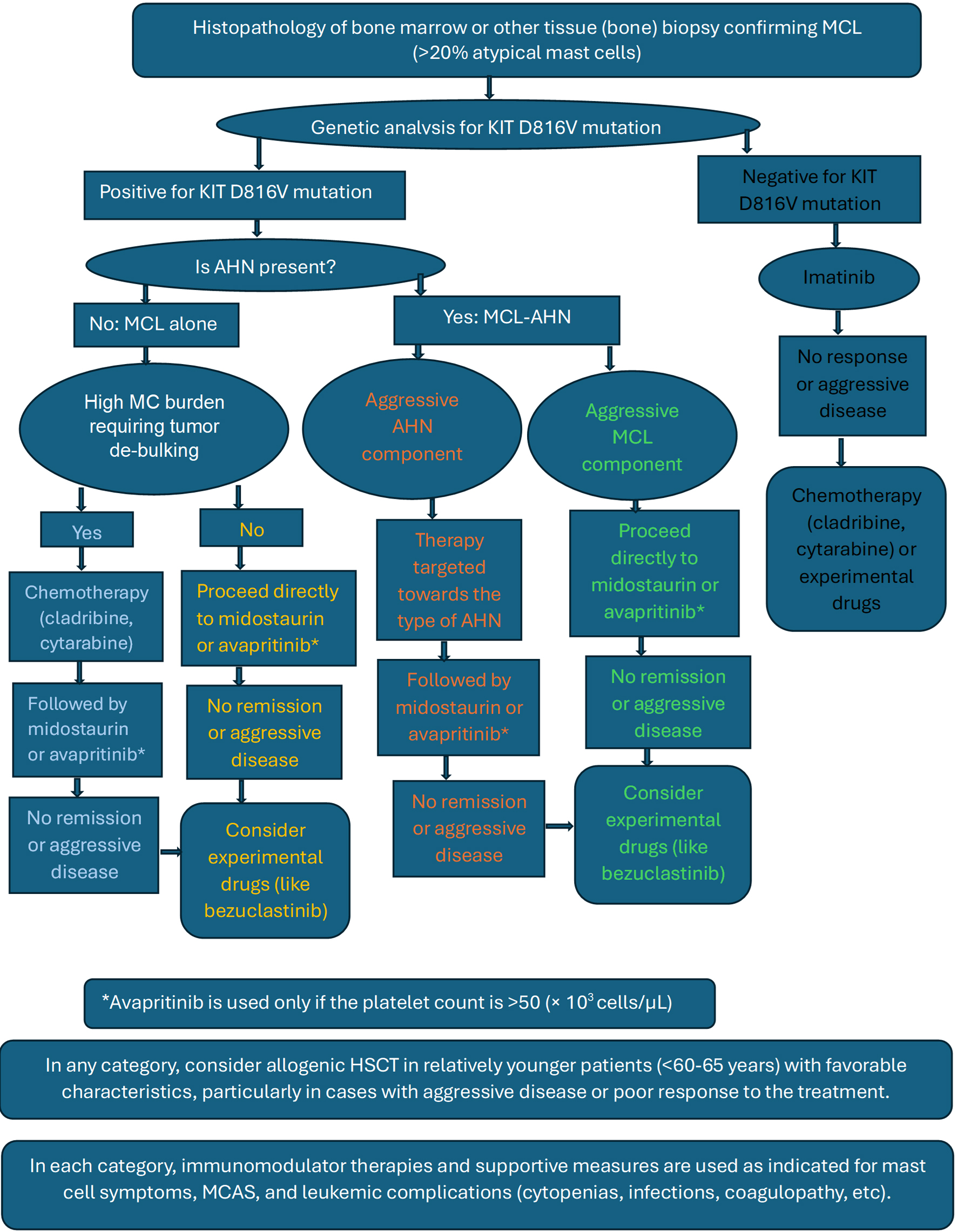
Tables
| Type | Key features | Diagnostic criteria | Subtypes | Prognosis |
|---|---|---|---|---|
| CM: cutaneous mastocytosis; ICC: International Consensus Classification; KIT: receptor tyrosine kinase type III; MC: mast cell; MCL: mast cell leukemia; MCS: mast cell sarcoma; SM: systemic mastocytosis; SM-AHN: SM with associated hematologic neoplasm; WHO: World Health Organization. | ||||
| CM | Confined to skin; no systemic organ involvement on biopsy or imaging | Clinical and dermatological findings: flushing, itching, urticaria, anaphylaxis; positive Darier’s sign | Maculopapular CM; urticaria pigmentosa; diffuse CM; cutaneous mastocytoma | Excellent; typically normal life expectancy |
| SM | Involves bone marrow or extracutaneous organs; may cause systemic symptoms and/or organ dysfunction | Requires 1 major + 1 minor OR ≥ 3 minor criteria: major: dense MC aggregates (> 15%); minor: atypical MCs, KIT mutation, CD25/CD2/CD30 expression, serum tryptase > 20 µg/L | Indolent SM; smoldering SM; aggressive SM; SM-AHN; MCL | Varies: indolent types have a good prognosis; aggressive/MCL subtypes have a poor prognosis |
| MCS | Rare, localized malignant tumor in soft tissue or visceral organ | Histology: highly atypical mast cells in an aggressive and destructive tumor. Typically KIT-mutated | None (distinct from systemic forms; may progress to MCL) | Very poor; rapid progression, often fatal |
| Subtype | Clinical features | Histopathology | Prognosis |
|---|---|---|---|
| ASM: aggressive systemic mastocytosis; BM: bone marrow; BMM: bone marrow mastocytosis; CMML: chronic myelomonocytic leukemia; ISM: indolent systemic mastocytosis; MC: mast cell; MCL: mast cell leukemia; MDS: myelodysplastic neoplasm; MPN: myeloproliferative neoplasm; SM-AHN: systemic mastocytosis-associated hematologic neoplasm; SSM: smoldering systemic mastocytosis. | |||
| ISM | Skin lesions, flushing, itching, abdominal pain, diarrhea, and anaphylaxis. No B-findings or C-findings. | Multifocal aggregates of mast cells in BM biopsy. | Normal life expectancy |
| BMM | All findings of ISM without skin lesions. | Serum tryptase < 125 µg/L. No dense MC infiltrates in an extramedullary organ. | Normal life expectancy |
| SSM | Higher MC burden than ISM. More severe symptoms than ISM with organ involvement but without organ damage. | Increased MC burden in BM. | Intermediate prognosis |
| SM-AHN | Associated with another hematologic disorder, such as MDS/MPN, or AML. CMML is the most common MDS/MPN. Symptoms of MC activation and cytopenias. | BM involvement with both MCs and an associated hematologic neoplasm. | Variable prognosis |
| ASM | Organ damage or dysfunction due to MC infiltration (C-findings): hepatomegaly, splenomegaly, lymphadenopathy, skeletal lesions, cytopenias, and malabsorption. | MC infiltration and organ damage in various tissues. | Poor prognosis |
| MCL | ASM+ rapid progression with > 20% MCs in BM aspirate. | BM infiltrated with > 20% MCs. | Poor prognosis |
| System/feature | Clinical findings | Notes |
|---|---|---|
| CNS: central nervous system; DIC: disseminated intravascular coagulation; GI: gastrointestinal; MCAS: mast cell activation syndrome; MCL: mast cell leukemia. | ||
| Skin | Blistering lesions | Common in secondary MCL |
| GI tract | Abdominal pain, diarrhea, peptic ulcers, GI bleeding, malabsorption | Also part of MCAS symptoms |
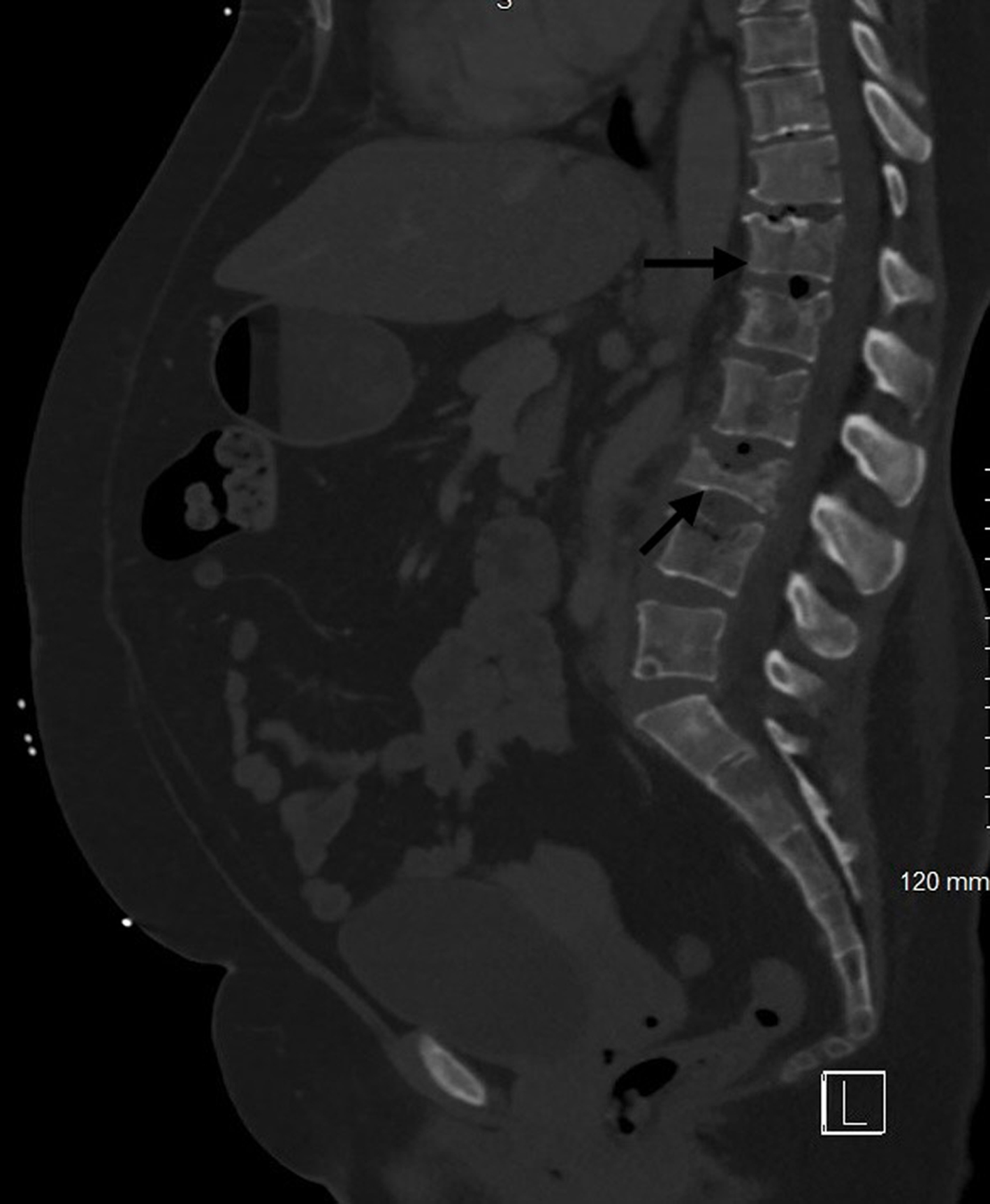
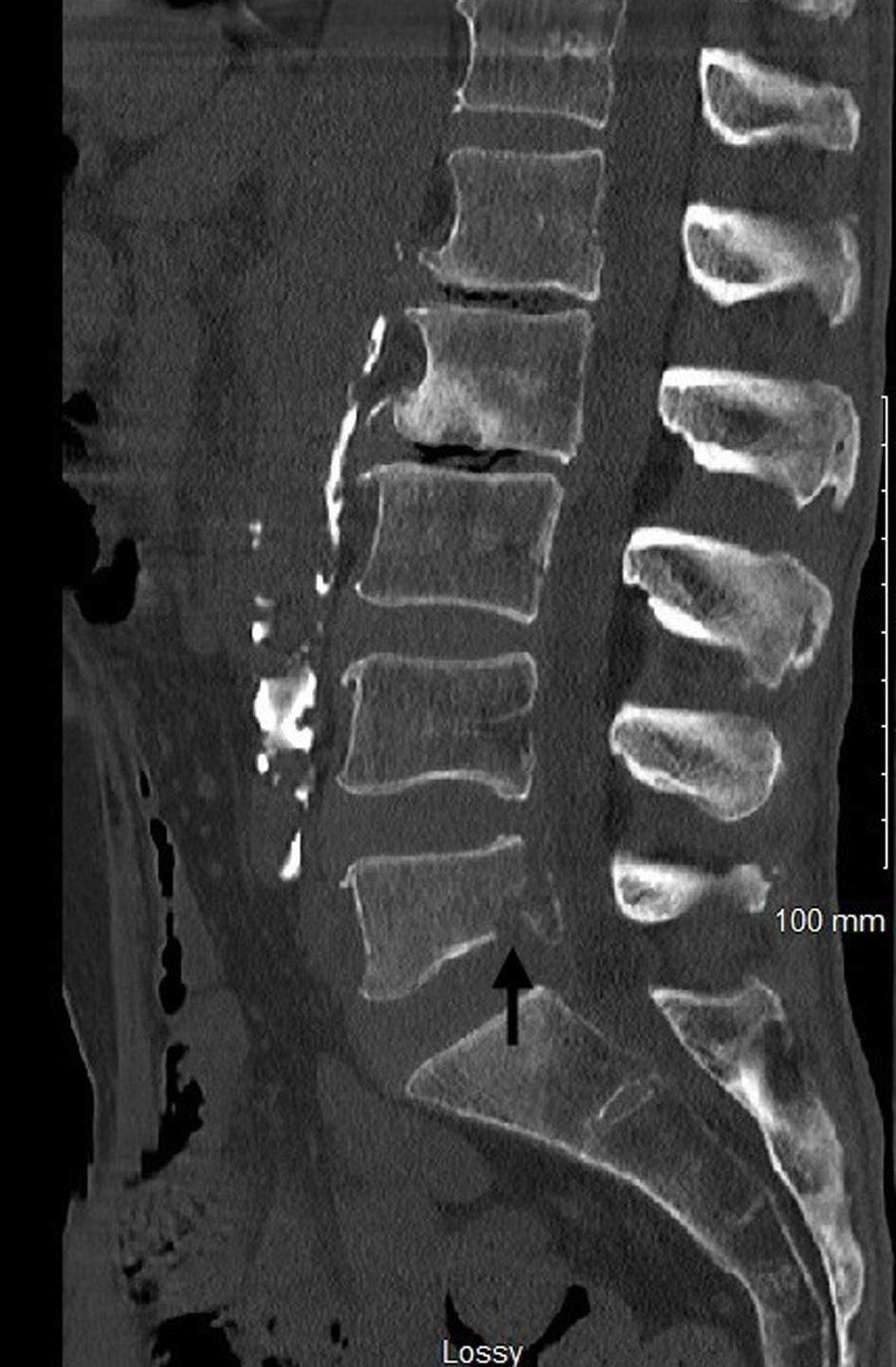
| Musculoskeletal system | Bone pain, osteolytic and osteoblastic lesions, pathological fractures, osteoporosis | Osteoblastic lesions less common; non-vertebral metastases rare |
| Bone marrow | Pancytopenia (anemia, thrombocytopenia, leukopenia), immunosuppression, infections, sepsis | Due to marrow infiltration by mast cells |
| CNS | Headache, dizziness, cognitive impairment, neuropsychiatric symptoms | CNS involvement is less frequent but clinically significant. |
| Liver (hepatomegaly) | Transaminitis, hypoalbuminemia, coagulopathy, DIC, ascites, portal hypertension | Liver dysfunction indicates advanced disease. |
| Spleen (splenomegaly) | Left upper quadrant pain/fullness, early satiety, hypersplenism with cytopenias | May accompany hepatomegaly |
| Lymph nodes | Diffuse lymphadenopathy; painful in some cases | Due to mast cell infiltration |
| Constitutional symptoms | Fever, fatigue, anorexia, malaise, weight loss, night sweats, decreased exercise tolerance | Common in chronic MCL |
| MCAS (systemic) | Anaphylaxis-like symptoms involving cardiovascular (hypotension, tachycardia, syncope), cutaneous (flushing, urticaria, pruritus), respiratory (wheezing, dyspnea, stridor), and GI (nausea, vomiting, diarrhea, abdominal pain) | It can occur at any stage/type of MCL. The best marker is elevated serum tryptase level. |
| Treatment category/drug | Role in management | Adverse/side effects | ||
|---|---|---|---|---|
| AdvSM: advanced systemic mastocytosis; AHN: associated hematological neoplasm; AIHA: autoimmune hemolytic anemia; ARDS: acute respiratory distress syndrome; CHF: congestive heart failure; FDA: Food and Drug Administration; GI: gastrointestinal; HSCT: hematopoietic stem cell transplantation; IFN-α: interferon-alpha; ILD: interstitial lung disease; ITP: immune thrombocytopenic purpura; MC: mast cell; MCAS: mast cell activation syndrome; MCL: mast cell leukemia; PUD: peptic ulcer disease; SM: systemic mastocytosis. | ||||
| Targeted therapy: tyrosine kinase inhibitors Mainstay of treatment currently. Midostaurin and avapritinib are the two agents approved by the FDA for MCL. | Midostaurin | Improved prognosis in all types of MCL irrespective of the stage of the disease. | Hyperglycemia; GI symptoms; myelosuppression (manageable); rare: arrhythmias, pneumonitis, hepatotoxicity | |
| Avapritinib (only used if platelets are > 50,000) | Good response rate even in the most aggressive forms of MCL. Favorable risk profile with fewer adverse effects when compared. | Pancytopenia; risk of intracranial bleeding (rare); peripheral and periorbital edema; GI issues; cognitive impairment | ||
| Imatinib | Mainly effective in patients without the KIT D816V mutation, some patients even achieving complete response. In patients with D816V mutation, only a partial response is noted. | Pancytopenia; fatigue, myalgias; GI symptoms; rare but serious: CHF, arrhythmias, hepatotoxicity, severe myelosuppression | ||
| Dasatinib | Only theoretical benefit noted in KIT D816V positive cases. Even in D816V negative cases, response rates and adverse effects were undesirable. | All the above adverse effects of imatinib +; rare: pulmonary arterial hypertension, pancreatitis, pleural effusion | ||
| Bezuclastinib | Drug in clinical trials with promising improvement in the disease markers and no serious adverse effects. | Pancytopenia; GI issues and taste disorders; rare and serious: hepatotoxicity, hypersensitivity reaction | ||
| Chemotherapy: Out of favor with the invention of targeted therapy. Tumor debulking to decrease MC burden and related symptoms. Pre- and post-HSCT to prolong remission and improve survival. Main treatment for aggressive AHN. | Cladribine | Potential value in other forms of AdvSM, but very little, and transient response seen in MCL. Used as a monotherapy or as a part of multidrug therapy for tumor debulking and before HSCT. | Adverse effects that are common to all chemo drugs: rash and pruritic; flu-like symptoms; GI symptoms; bone marrow suppression with increased risk of infections; hepatotoxicity (transaminitis); fatigue and malaise; tumor lysis syndrome | Arrhythmias; increased risk of AHN |
| Cytarabine | Used in combination with cladribine for tumor debulking and with fludarabine before HSCT. | Cerebellar toxicity; peripheral neuropathy; rare: cytarabine syndrome, ARDS, pancreatitis | ||
| Fludarabine | Used as a part of polychemotherapy with either cladribine or cytarabine, mainly before HSCT. | Encephalopathy/coma and seizures; AIHA and ITP; heart failure; ILD and pulmonary fibrosis | ||
| IFN-α | Limited role in MCL. Studied in combination with steroids. | Psychiatric issues; thyroid dysfunction, alopecia; peripheral neuropathy; ILD and pulmonary fibrosis (rare) | ||
| Immuno-modulators: No disease-modifying effect. Used to manage MC-related symptoms alongside chemotherapy or targeted therapies. Commonly employed in the treatment of MCAS. | Thalidomide | Can be used as a single therapy for benign forms of SM. Not well studied in AdvSM/MCL; can be used to control MC symptoms. | Drowsiness and sedation; peripheral neuropathy; pancytopenia | |
| Cromolyn | Control symptoms and improve quality of life in all forms of SM. Important role in MCAS. | Rash and pruritus; dizziness; GI symptoms (nausea, vomiting, diarrhea); paradoxical bronchospasm; fatigue and malaise | ||
| Cortico-steroids | Main role in refractory and severe (anaphylaxis) cases of MCAS. Control of musculoskeletal pain associated with bone metastases. Used in organ damage from MC infiltration, particularly GI issues. | Typically, not used long enough to cause adverse effects: muscle weakness; Cushingoid features; PUD and GI bleeding; increased risk of infections; vision changes, headache, and psychosis | ||