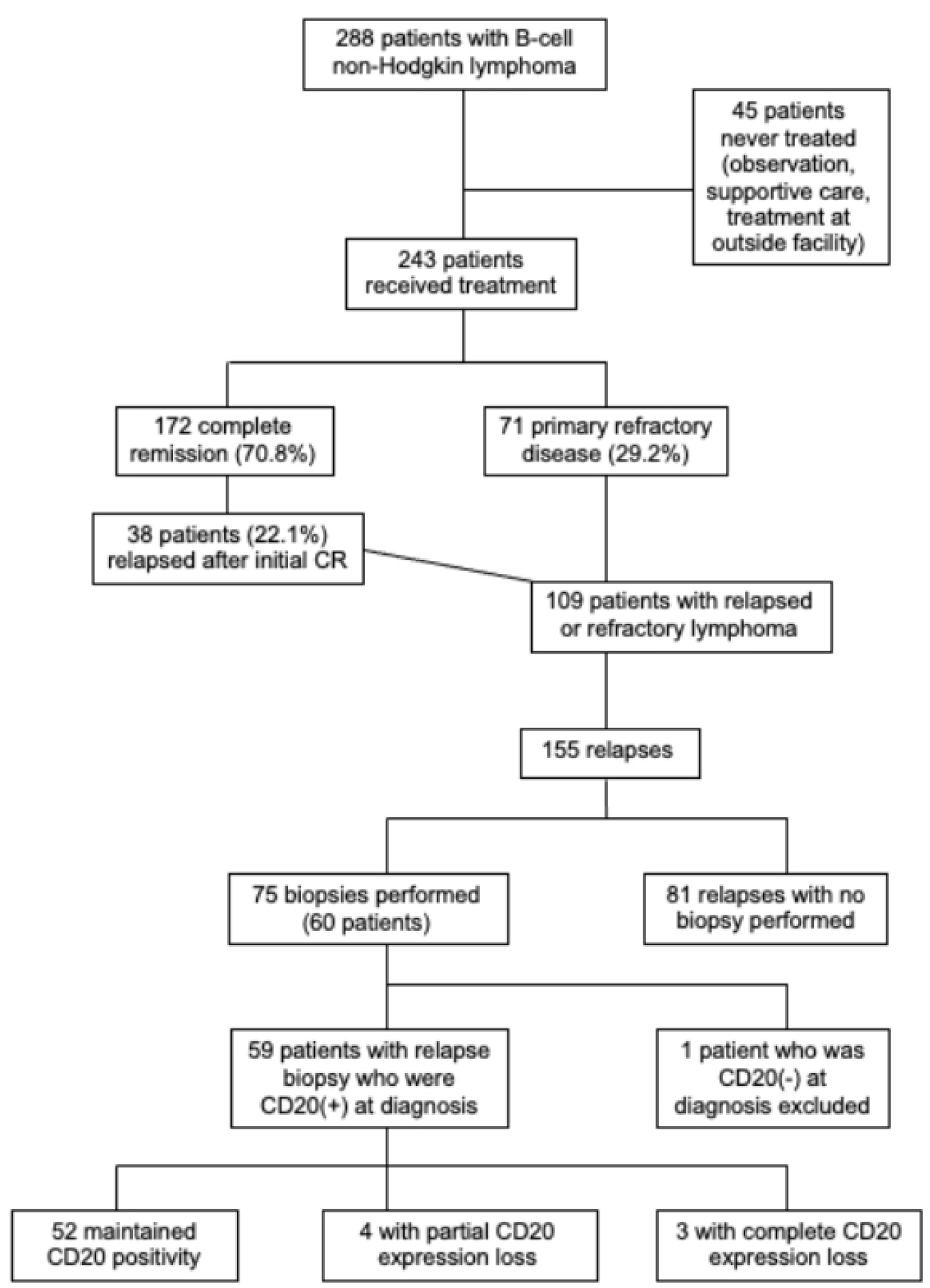
Figure 1. Flow diagram of patient eligibility.
| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 13, Number 6, December 2024, pages 268-277
Retrospective Study of CD20 Expression Loss in Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma
Figures

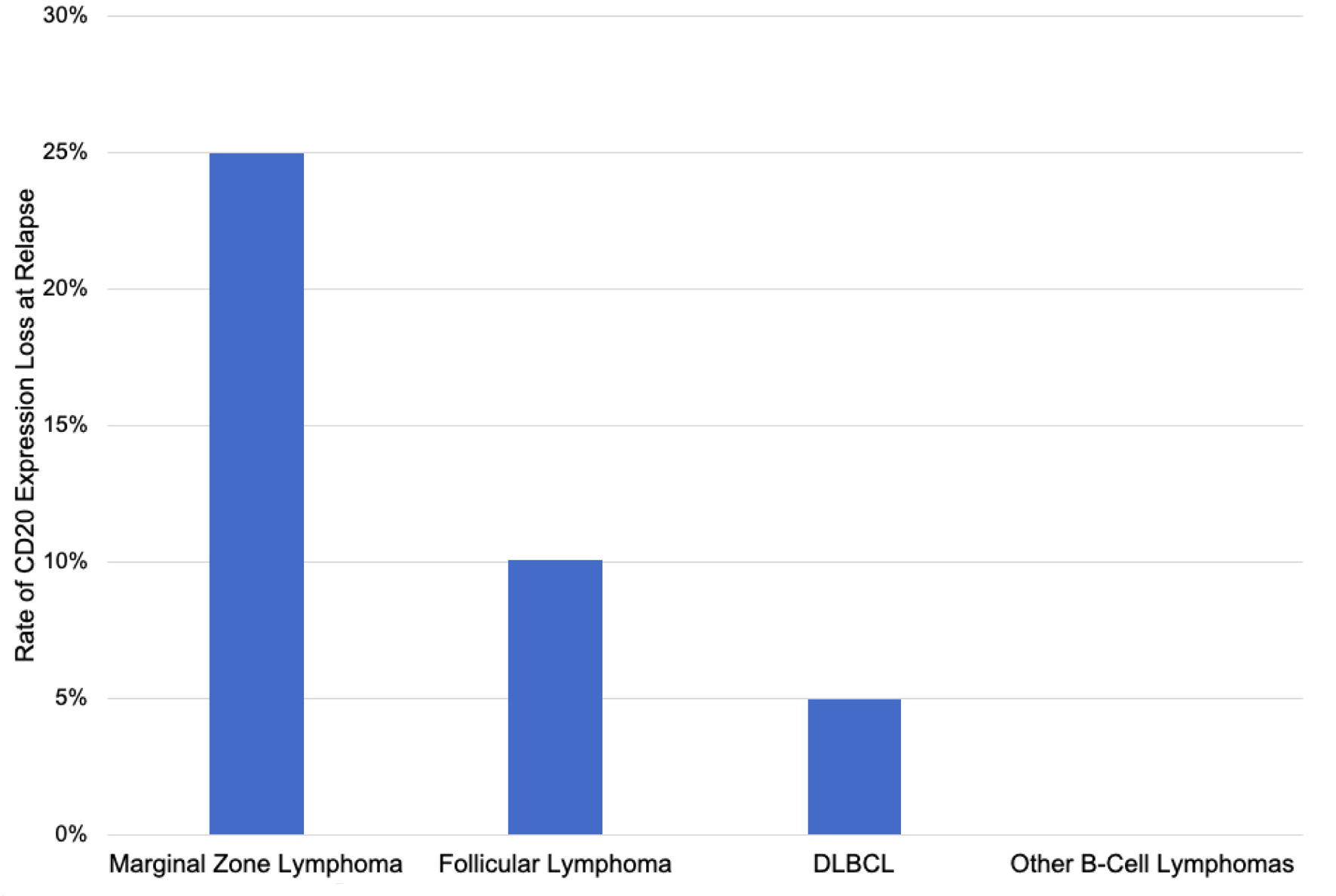
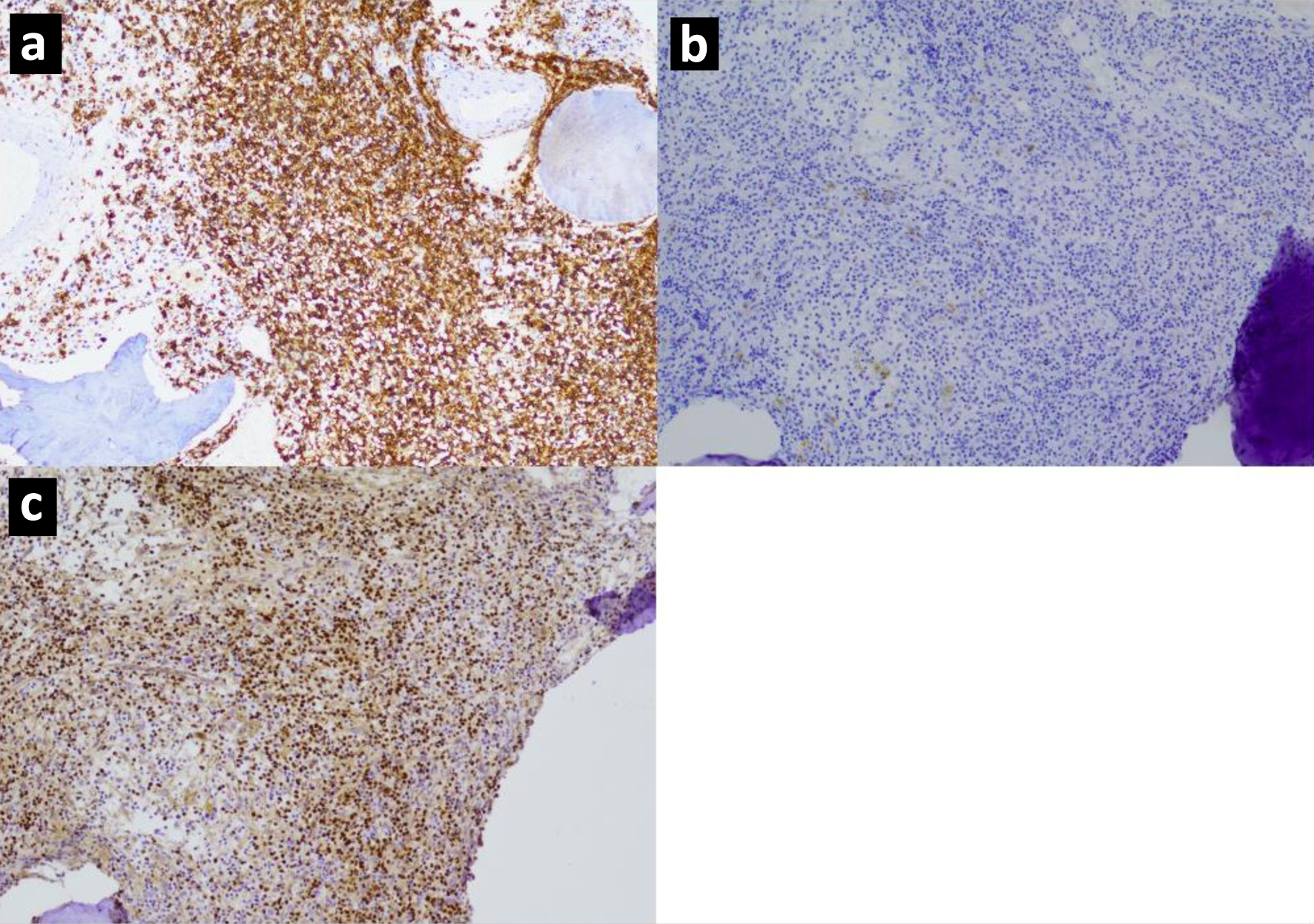
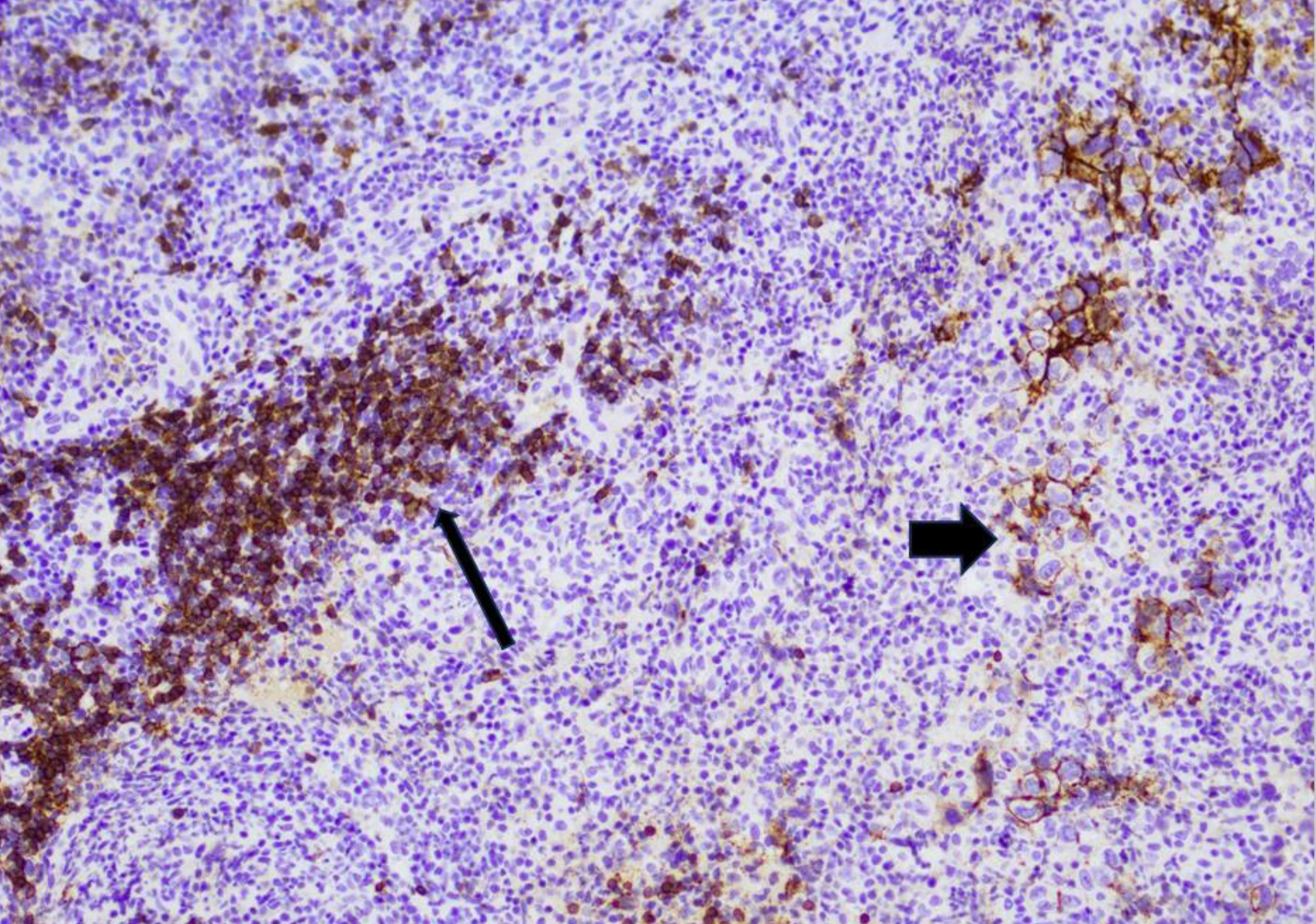
Tables
| Demographics | |
|---|---|
| CNS: central nervous system. | |
| Gender | |
| Males | 144 (59.3%) |
| Females | 99 (40.7%) |
| Age at diagnosis (years) | |
| Median | 56 |
| Range | 19 - 94 |
| Ethnicity/race | |
| Hispanic | 160 (65.9%) |
| White/European | 34 (14.0%) |
| Black/African-American | 27 (11.1%) |
| Asian | 15 (6.2%) |
| Other | 7 (2.9%) |
| Stage | |
| Stage I | 27 (11.1%) |
| Stage II | 55 (22.6%) |
| Stage III | 28 (11.5%) |
| Stage IV | 111 (45.7%) |
| Primary CNS | 5 (2.1%) |
| Unknown | 17 (7.0%) |
| Number of patients (% cohort) | CRR to first-line treatment | Primary refractory diseasea | Relapse after CRb | Relapsed/refractory | Number of patients with biopsy at relapse (% cohort) | |
|---|---|---|---|---|---|---|
| aPrimary refractory disease includes patients with partial remission, stable disease, or progressive disease after first-line treatment. bAmong patients with initial CR to first-line treatment. cDouble expressor, triple expressor, or HGBCL-NOS. CR: complete remission; CRR: complete remission rate; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HGBCL: high-grade B-cell lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; NOS: not otherwise specified; PCNSL: primary central nervous system lymphoma; PMBCL: primary mediastinal B-cell lymphoma. | ||||||
| Overall | 243 | 70.8% | 29.2% | 22.1% | 44.9% | 60 |
| DLBCL | 127 (52.3%) | 80.3% | 19.7% | 16.7% | 33.1% | 20 (33.3%) |
| FL | 34 (14.0%) | 76.5% | 23.5% | 46.2% | 58.9% | 18 (30.0%) |
| MZL | 18 (7.4%) | 55.6% | 44.4% | 40.0% | 66.7% | 10 (16.7%) |
| HGBCLc | 16 (6.6%) | 25.0% | 75.0% | 0% | 75.0% | 3 (5.0%) |
| MCL | 11 (4.5%) | 36.4% | 63.6% | 50.0% | 81.2% | 6 (10.0%) |
| PCNSL | 5 (2.1%) | 40.0% | 60.0% | 50.0% | 80.0% | 1 (1.7%) |
| PMBCL | 4 (1.6%) | 100% | 0% | 25.0% | 25.0% | 1 (1.7%) |
| Burkitt | 4 (1.6%) | 75.0% | 25.0% | 0% | 25.0% | 0 (0%) |
| Plasmablastic | 2 (1.6%) | 50.0% | 50.0% | 100% | 100% | 1 (1.7%) |
| NOS/other | 22 (9.1%) | 69.6% | 30.4% | 0% | 30.4% | 0 (0%) |
| CD20 expression | First relapse (n = 52) | Second relapse (n = 13) | Third relapse (n = 4) | Fourth relapse (n = 3) | Fifth relapse (n = 2) |
|---|---|---|---|---|---|
| Positive | 48 | 11 | 4 | 3 | 1 |
| Partial | 3 | 1 | 0 | 0 | 0 |
| Negative | 1 | 1 | 0 | 0 | 1 |
| Rate of CD20 expression loss | 7.7% | 15.4% | 0% | 0% | 50% |
| Patient and lymphoma characteristics | Biopsy at diagnosis | First-line treatment | First relapse biopsy and treatment | Second relapse biopsy and treatment | Third relapse and later |
|---|---|---|---|---|---|
| BR: bendamustine + rituximab; CR: complete remission; DAREPOCH: dose-adjusted rituximab + etoposide + prednisone + vincristine + cyclophosphamide + doxorubicin; DLBCL: diffuse large B-cell lymphoma; F: female; FCR: fludarabine + cyclophosphamide + rituximab; Flow: flow cytometry; FR: fludarabine + rituximab; HGBCL: high-grade B-cell lymphoma; ICE: ifosfamide + carboplatin + etoposide; M: male; PR: partial remission; PET: positron emission tomography; PD: progression of disease; R1: first relapse; R2: second relapse; R3: third relapse; R4: fourth relapse; R5: fifth relapse; RCHOP: rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone; RICE: rituximab + ifosfamide + carboplatin + etoposide; SD: stable disease; SCT: stem cell transplant. | |||||
| 1) 51M DLBCL ABC type Stage IV Partial CD20 loss at R1 | Lymph node: IHC CD20(+) | RCHOP => CR | Lymph node: IHC CD20(+), Flow CD20(+) dim RICE => PD | Death 3 months after R1 | |
| 2) 33M DLBCL ABC type Stage IV Partial CD20 loss at R1 | Lymph node: IHC CD20(+) | RCHOP => CR | Lymph node: IHC CD20(+) weak RICE => PR Autologous SCT => CR | Alive 70 months after R1 | |
| 3) 47M Follicular Stage IV CD20 loss at R1 | Bone marrow: IHC CD20(+), Flow CD20(+) Chest wall mass: IHC CD20(+), Flow CD20(+) | RCHOP + rituximab maintenance => PD | Lymph node: IHC CD20(-), transformation to HGBCL RICE => PD | Death 3 months after R1 | |
| 4) 54F Marginal zone Stage IV Partial CD20 loss at R1 | Bone marrow: IHC CD20(+), Flow CD20(+) | Rituximab => PD | Bone marrow: IHC CD20(-), Flow CD20(+) Ibrutinib => PD | Death 4 months after R1 | |
| 5) 56F Follicular Stage IV CD20 loss at R2 | Lymph node: IHC CD20(+) Bone marrow: IHC CD20(+) | RCHOP + rituximab maintenance => CR | No biopsy, relapse on PET BR => PR | Lymph node: IHC CD20(-), transformation to DLBCL Peripheral blood: Flow CD20(-), no evidence of transformation ICE => PD | Death 3 months after R2 |
| 6) 58M Marginal zone Stage III Partial CD20 loss at R2 | Orbital mass: IHC CD20(+) | FCR => CR | Biopsy results unavailable FCR => CR | Lymph node: IHC CD20(-), Flow CD20(+) dim, transformation to DLBCL RCHOP => PD | Death 5 months after R2 |
| 7) 60M Marginal zone Stage IV CD20 loss at R5 | Peripheral blood: IHC CD20(+), Flow CD20(+) Bone marrow: IHC CD20(+), Flow CD20(+) | RCHOP => PR | Bone marrow: IHC CD20(+), Flow CD20(+) FR => PR | Peripheral blood: Flow CD20(+) Spleen: IHC CD20(+), Flow CD20(+) Idelalisib + splenectomy => SD | R3: Peripheral blood: Flow CD20(+) Ibrutinib => PD R4: Peripheral blood: Flow CD20(+) Bone marrow: IHC CD20(+) Bendamustine/ofatumumab + ofatumumab maintenance => PD R5: Peripheral blood: Flow CD20(-) Abdominal wall mass: IHC CD20(-), Flow CD20(-) Duodenal mass: IHC CD20(-) ICE => PD Death 4 months after R5 |