Efficacy and Safety of Daratumumab, Lenalidomide and Dexamethasone Therapy in the First Relapse of Multiple Myeloma Patients – Real World Data from Hungary
DOI:
https://doi.org/10.14740/jh2142Keywords:
Multiple myeloma, Daratumumab, Lenalidomide, Treatment response, Progression-free survival, Overal survival, FrailtyAbstract
Background: Daratumumab is a monoclonal antibody targeting CD38, which has demonstrated efficacy in both newly diagnosed and relapsed or refractory multiple myeloma (MM). The objective of our study was to evaluate the effectiveness and safety of the daratumumab, lenalidomide, and dexamethasone (D-Rd) regimen as a second-line therapy in a real-world clinical setting.
Methods: A total of 55 Hungarian patients with MM were enrolled, all of whom received D-Rd at first relapse through a special reimbursement program between February 2022 and August 2023. Treatment responses, progression-free survival (PFS), and overall survival (OS) were assessed in the intent-to-treat population as well as in selected subgroups. Safety outcomes were also systematically collected.
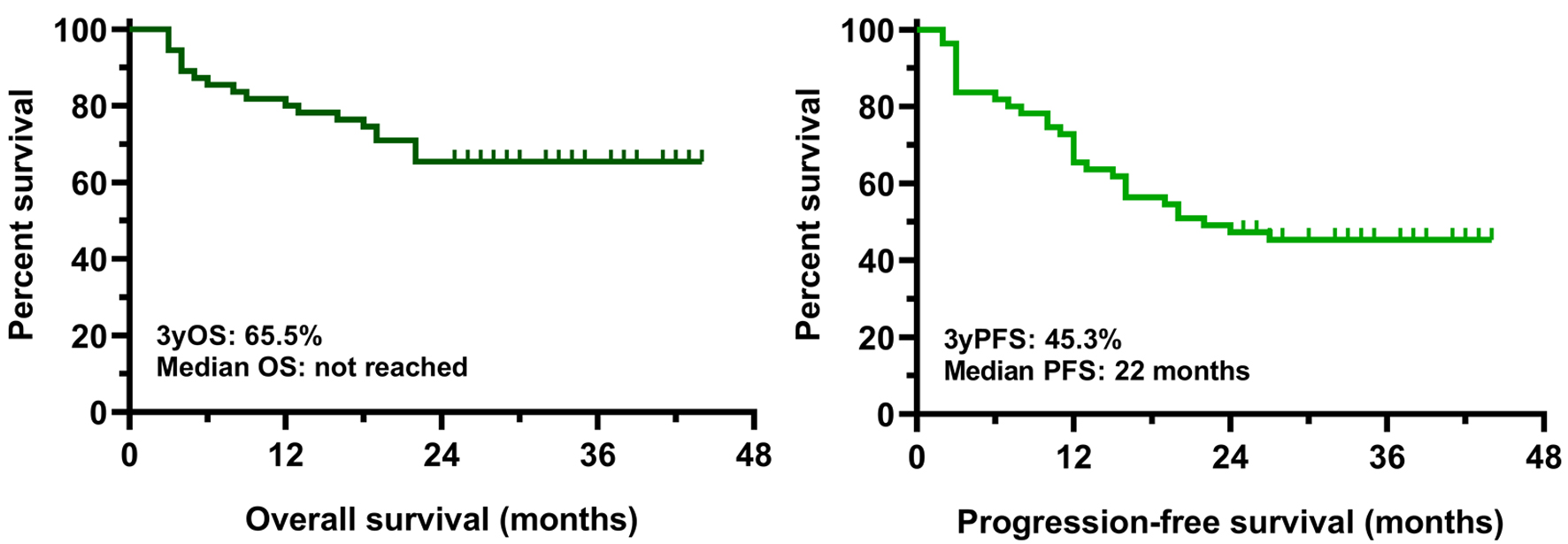
Results: Treatment response was observed in 49 patients (89%), with 12 complete responses, 22 very good partial responses, and 15 partial responses. After a median follow-up of 36.6 months, the median PFS for the entire cohort was 22.0 months, while median OS had not been reached yet. Patients with advanced-stage disease (Revised International Staging System stage 3) showed significantly poorer survival outcomes compared with those in stage 1 or 2 (PFS: P < 0.001; OS: P = 0.015). High-risk cytogenetic abnormalities and frailty were also associated with markedly inferior prognosis. In contrast, neither prior autologous stem cell transplantation nor lenalidomide maintenance therapy had a significant impact on survival. During the study period, three deaths due to severe infections occurred, while the most common adverse events were mild hematologic toxicities and injection-related reactions.
Conclusions: Our findings support the use of D-Rd as an effective option for first relapse in MM patients. However, our survival data are inferior to the results of the pivotal studies targeting the same population.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








