Iron-Induced Hypophosphatemia: A Review of Pathophysiology, Drug Safety, and Pharmacogenomic Perspectives
DOI:
https://doi.org/10.14740/jh2111Keywords:
Ferric carboxymaltose, Hypophosphatemia, FGF23, Drug safety, Pharmacogenomics, Adverse drug reaction, Iron deficiency anemia, HematologyAbstract
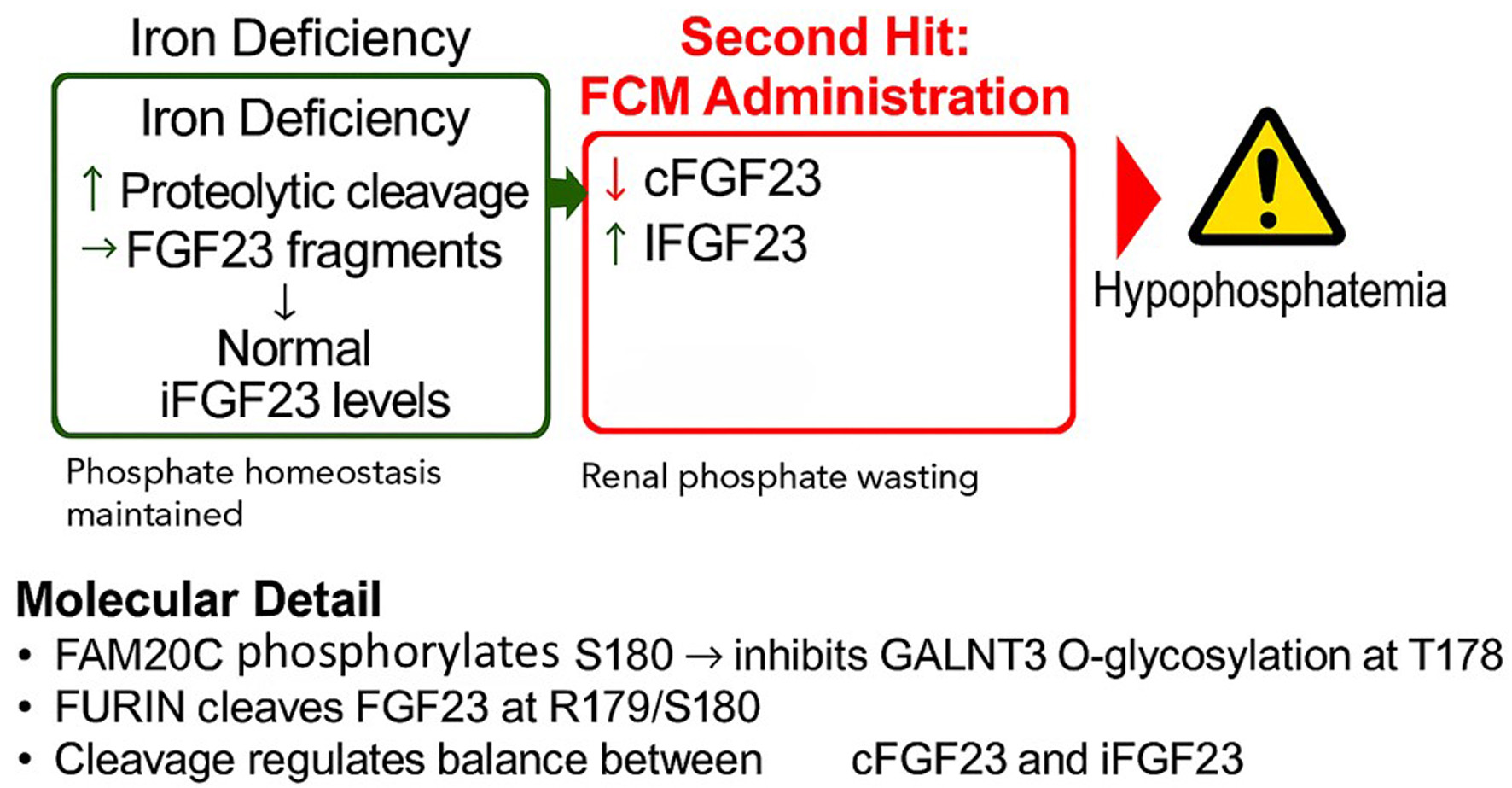
Intravenous (IV) iron therapy is a cornerstone in the management of iron deficiency anemia, yet its administration is associated with significant side effects, most notably hypophosphatemia. This adverse event is not a class effect but is disproportionately linked to specific formulations, particularly ferric carboxymaltose (FCM). This review synthesizes the current clinical evidence, elucidates the central pathophysiological role of fibroblast growth factor 23 (FGF23), and explores a potential pharmacogenomic basis for individual susceptibility. Clinical data from numerous randomized controlled trials and meta-analyses confirm that FCM induces hypophosphatemia in over 50% of patients, a rate far exceeding that of other IV iron preparations. The proposed underlying mechanism involves a “two-hit” process: pre-existing iron deficiency upregulates FGF23 gene transcription, while FCM administration is thought to inhibit the proteolytic cleavage of the FGF23 protein. This uncoupling of production and degradation leads to a surge in active, intact FGF23 (iFGF23), causing renal phosphate wasting. We explore the potential for genetic polymorphisms in FGF23 and its key processing enzymes, such as FURIN, GALNT3, and FAM20C, to modulate individual risk. Understanding this complex interplay is crucial for risk stratification, appropriate formulation selection, and patient monitoring to mitigate the acute and chronic consequences of iatrogenic hypophosphatemia, including debilitating fatigue and osteomalacia.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.








