| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Review
Volume 000, Number 000, August 2025, pages 000-000
Novel Agents and Immunotherapies for Primary Diffuse Large B-Cell Lymphoma of the Central Nervous System: Innovating for Impact in a Disease With Unmet Needs
National Institutes of Health, Bethesda, MD 20892, USA
Manuscript submitted June 4, 2025, accepted August 8, 2025, published online August 27, 2025
Short title: Novel Therapies for PCNS-DLBCL
doi: https://doi.org/10.14740/jh2095
- Abstract
- Introduction
- PCNSL Pathophysiology
- Treatment of PCNSL
- Conclusion and Future Directions
- References
| Abstract | ▴Top |
Primary diffuse large B-cell lymphoma of the central nervous system (CNS-DLBCL), the most prevalent subtype of primary CNS lymphoma (PCNSL), is a highly aggressive extranodal non-Hodgkin lymphoma (NHL) that arises in the brain, spinal cord, leptomeninges, and orbits. Systemic methotrexate-based chemoimmunotherapy regimens, followed by consolidative autologous stem cell transplantation (ASCT), are the standard treatments for newly diagnosed PCNSL. A considerable number of patients with PCNSL, however, are medically frail and possess multiple comorbidities, which render them unsuitable for these intensive treatments. Furthermore, a substantial proportion of those who undergo treatment still face the challenge of disease recurrence. There is no universally accepted treatment for relapsed disease, particularly for patients who are unable to tolerate systemic chemotherapy, and participation in clinical trials is encouraged. There is a notable treatment gap in PCNSL, underscoring an urgent need to investigate novel agents and immunotherapies that could potentially offer superior tolerability and efficacy profiles. Emerging therapies could play a pivotal role in expanding the therapeutic landscape and addressing the limitations inherent in current treatments. This review examines the oncogenesis of PCNSL, highlighting its reliance on chronic active B-cell receptor (BCR) and nuclear factor-kappa B (NF-κB) signaling pathways, mechanisms of immune evasion, and characteristics of its immunosuppressive tumor microenvironment (TME), which have facilitated the exploration of methotrexate-free targeted therapies for this disease.
Keywords: Primary CNS lymphoma; BTK inhibitors; Immunomodulatory drugs; Immunotherapy
| Introduction | ▴Top |
Primary large B-cell lymphoma of immune-privileged sites (IP-LBCL) encompasses a category of highly aggressive extranodal non-Hodgkin lymphomas (NHL) that originate in the central nervous system (CNS), vitreoretinal compartment, and testes of immunocompetent individuals [1]. When IP-LBCL is confined to the CNS, it is clinically termed primary central nervous system lymphoma (PCNSL). Diffuse large B-cell lymphoma of the CNS (CNS-DLBCL) is the most prevalent subtype of PCNSL [2]. PCNSL accounts for approximately 4% of all primary brain tumors and its incidence increases with age, peaking in individuals over 65 years [3]. The treatment options for PCNSL are limited. Broad consensus exists to include high-dose methotrexate (HD-MTX) as the backbone of polychemotherapy induction regimens [4]. Consolidative therapies include high-dose chemotherapy and autologous stem cell transplantation (ASCT) or, for less medically fit patients, whole-brain radiotherapy (WBRT) [5]. For patients over 60 years of age, WBRT is generally not advised due to the potential for severe neurotoxic side effects, and cytarabine (Ara-C) is often chosen as the consolidation therapy, provided it was not utilized during the induction phase [6].
PCNSL has a poor prognosis, with a survival rate of two months if left untreated [7]. MTX-based chemotherapy extended the median overall survival (OS) to 30 - 60 months [8]. Although a complete response (CR) and progression-free survival (PFS) of approximately 50% can be achieved for individuals who can complete treatment with a regimen such as MATRix (HD-MTX, cytarabine, thiotepa, rituximab), followed by consolidation with ASCT or WBRT [9], a substantial portion of patients still experience relapse of PCNSL. The median OS for those with relapsed disease is six months. While most relapses generally occur within the first two years of diagnosis, relapse can occur after 10 years [10]. Since there is an absence of a universally accepted standard treatment for relapsed or refractory (R/R) disease, it is recommended that patients enroll in clinical trials. This review discusses how the improved understanding of the cell-of-origin (COO) of PCNSL, its oncogenic reliance on chronic active B-cell receptor (BCR) and nuclear factor-kappa B (NF-κB) signaling, mechanisms of immune evasion, and its immunosuppressive tumor microenvironment (TME) has created the opportunity to study targeted therapies for PCNSL. Although these agents are mostly studied in R/R disease, the unique biology of PCNSL lends itself to the potential of these drugs being investigated in the frontline setting.
| PCNSL Pathophysiology | ▴Top |
Cell-of-origin (COO)
PCNSL is of activated B-cell (ABC) origin, frequently negative for CD10, although CD10 is detectable in 10-20% of cases [11], and positive for interferon regulatory factor 4 (IRF4)/multiple myeloma oncogene 1 (MUM1) [12]. These cells, derived from the post-germinal center (GC), have B-cell marker expression of CD19, CD20, CD79a and CD79b [13]. Gene expression profiling confirms that PCNSL shares key features with the MYD88/CD79B-mutated (MCD) genetic subtype of DLBCL, marked by chronic active BCR signaling and inactivating mutations in genes encoding class 1 and 2 human leukocyte antigens (HLA) [14]. Gain-of-function mutations in genes encoding the myeloid differentiation primary response 88 (MYD88), which functions as an adaptor protein for toll-like receptor (TLR) signaling pathways, and the BCR subunit CD79B, are frequently observed mutations across MCD-DLBCL. A hallmark feature of MCD-DLBCL is the constitutive activation of the downstream gene expression regulator NF-κB [15]. These characteristics of the MCD genetic subtype of DLBCL are especially prevalent in PCNSL [16].
The MYD88, TLR9, and BCR (My-T-BCR) complex co-localizes with mammalian target of rapamycin (mTOR) and drives constitutive NF-κB signaling, which results in expression of anti-apoptotic and pro-proliferation genes that are key to the survival of PCNSL cells [17]. Bruton’s tyrosine kinase (BTK) serves as a critical intermediary linking the BCR and TLR pathways to NF-κB, thereby positioning this protein as a potential therapeutic target in PCNSL. Recognition of self-antigens is another factor that drives persistent activation of BCR, and the oncogenesis of MCD-DLBCL and PCNSL [18]. B cells in PCNSL display rearranged and heavily somatically mutated immunoglobulin genes with a limited repertoire of immunoglobulin heavy chain genes, suggesting an antigen-driven component in chronic BCR activation [19]. Among the neuron-derived proteins recognized by BCRs in PCNSL are the physiologically expressed GRINL1A, ADAP2, galectin-3, and BAIAP2 [20]. This implies that resident CNS cell populations act as antigenic stimuli for clonal B cells in PCNSL. A proof-of-concept study identified post-translationally modified CNS autoantigens as drivers of chronic antigenic stimulation in PCNSL. Atypically hyper-N-glycosylated sterile α-motif domain containing protein 14 (SAMD14) and neural tissue-specific F-actin-binding protein I (neurabin-I) were identified as autoantigen targets of eight out of 12 BCRs in PCNSL [21].
Tumor microenvironment (TME)
The TME is composed of a tumor’s ecosystem that consists of immune cells, fibroblasts, extracellular matrix, cytokines, and growth factors which either promote or inhibit tumor growth [22]. In the case of PCNSL, the TME is skewed toward being immunosuppressive, thereby restricting the function of immune surveillance cells. Specifically, there is a reduction in the presentation of cancer antigens to T cells through the suppression of major histocompatibility complex (MHC) expression on antigen-presenting cells, and an inhibition of cytotoxic T-cell activity through the amplification of chromosome 9p24.1, which encodes the inhibitory programmed death-ligand proteins PD-L1 and PD-L2 on resident cells within the TME [23]. The presence of M2 macrophages, which recruit IL-10-secreting regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), further promote an immunosuppressive TME [24]. Immune checkpoint inhibitors (ICIs), therefore, hold the potential to counteract these immunosuppressive properties. A significant challenge in PCNSL, however, is the described heterogeneity in immune checkpoint expression. The reported frequency of PD-L1 expression in PCNSL tissue is highly variable, ranging from 4.1% to 97% [25]. Moreover, the TME is a dynamic system, subject to remodeling based on the tumor stage, surrounding cellular composition, and signaling milieu, thereby potentially limiting the impact of ICIs in PCNSL [26].
| Treatment of PCNSL | ▴Top |
Current frontline treatment of PCNSL
While anthracycline-based chemotherapy is fundamental to curative treatment for systemic DLBCL, it has significantly reduced efficacy in PCNSL and this is partly attributable to the chemotherapy’s inability to consistently penetrate the blood-brain barrier (BBB), resulting in low CNS bioavailability of the drug [27]. Chemotherapies that incorporate HD-MTX, an agent that reliably crosses the BBB, are the frontline treatments for PCNSL, but these therapies are associated with significant toxicities that limit their use in the medically frail. MTX-based chemotherapy regimens also incorporate rituximab, the anti-CD20 monoclonal antibody. Examples of such regimens are MATRix [28], MTR (HD-MTX, temozolomide, rituximab) [29], and R-MVP (rituximab, HD-MTX, vincristine and procarbazine) [30]. Successful induction with these chemoimmunotherapy regimens still requires consolidation with high-dose chemotherapy and ASCT that is typically suitable for younger and medically fit patients. There is currently no standard therapy for relapsed PCNSL, and patients with relapsed disease are encouraged to participate in clinical trials.
Limitations of systemic chemoimmunotherapy
The addition of rituximab to chemotherapy has transformed the treatment landscape of systemic B-cell lymphoma, but its benefit in PCNSL is still unclear [31]. Rituximab has limited CNS permeability, with CSF concentrations ranging from 0.1% to 4% of its serum concentration, possibly in part due to its large molecular weight of 145 kDa [32]. While retrospective studies have suggested improved outcomes with the addition of rituximab to chemotherapy in PCNSL [33-35], results of prospective trials investigating the efficacy of rituximab in PCNSL have been conflicting. The largest randomized controlled trials to date, the HOVON 105/ALLG NHL 24 [36] and the IELSG32 [28], have shown conflicting results regarding the clinical efficacy of rituximab in the treatment of PCNSL (Tables 1 and 2) [28, 36-44].
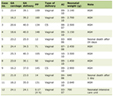 Click to view | Table 1. Prospective Clinical Trials of Targeted Therapies: Endpoints |
 Click to view | Table 2. Prospective Clinical Trials of Targeted Therapies: Summary of Outcomes |
Subsequent to induction, patients are advised to undergo high-dose chemotherapy followed by ASCT. WBRT (45 Gy) is considered for patients who are not candidates for ASCT, although this therapy poses a substantial risk of neurotoxicity [45]. Reduced-dose (approximately 25 Gy) WBRT as consolidation for MTX-based induction chemotherapy has also demonstrated a role in inducing responses in PCNSL, but with less associated neurotoxicity [46]. The limitations inherent in current frontline treatments for PCNSL, along with the lack of standardized therapies for R/R disease, present an opportunity to investigate targeted agents for PCNSL.
BTK inhibitors (BTKi)
The role of BTK as an intermediary in the signaling pathway between BCR and NF-κB is the rationale for the investigation of BTKi in PCNSL [12, 47]. Ibrutinib is a first-in-class BTKi. The pharmacokinetics of ibrutinib and its ability to cross the BBB have been investigated and, when corrected for protein binding of 97%, the CSF to plasma ratio was reported to be 28.7%, indicating that a significant portion of the free drug penetrates the BBB [37]. While ibrutinib demonstrated clinically effective concentrations in the CSF and inhibition of chronic active BCR signaling, the duration of response to ibrutinib as a monotherapy is brief [37]. Grommes et al reported findings from a phase 1 study of ibrutinib monotherapy in 13 patients with R/R PCNSL. Clinical responses were observed in 10 of the 12 evaluable patients, with five achieving CR. The median PFS with single agent ibrutinib was 4.6 months, and the median OS was 15 months [38]. Soussain et al reported comparable survival outcomes of a phase 2 trial of ibrutinib monotherapy in patients with R/R PCNSL and primary vitreoretinal lymphoma, with PFS and OS rates of 4.8 and 19.2 months, respectively. Two patients experienced pulmonary aspergillosis, one with a fatal outcome [39]. A second-generation BTKi has also been investigated in PCNSL. A phase 1/2 study of tirabrutinib in 44 patients with R/R PCNSL demonstrated an overall response rate (ORR) of 64%. However, with a median PFS of 2.9 months, the response duration with this newer generation BTKi remains limited [40].
The incorporation of ibrutinib into an anthracycline-based chemotherapy regimen, as reported by Lionakis et al, resulted in more sustained remissions [37]. This was demonstrated by a phase 1b study of 18 patients with R/R PCNSL treated with dose-adjusted temozolomide, etoposide, liposomal doxorubicin, dexamethasone, ibrutinib, and rituximab (DA-TEDDi-R). Among the 14 evaluable patients of whom 12 (86%) achieved either a CR or a complete response unconfirmed (CRu), the median PFS was 15 months and a median OS was not reached, with 51% of patients alive at 1 year. Increased incidence of aspergillosis was noted with this regimen (Tables 1 and 2) [37-44].
Orelabrutinib is an irreversible second-generation BTKi that has greater bioavailability and fewer adverse effects than ibrutinib [48]. A retrospective study evaluated the efficacy of orelabrutinib (150 mg/day) and rituximab (250 mg/m2 per week) (RO cohort), versus orelabrutinib alone (100 mg twice a day) (OB cohort) and ibrutinib alone (560 mg/day) (IB cohort) among patients with R/R PCNSL. The ORR was higher for patients in the RO cohort than in the IB cohort, and the PFS and OS rates were higher in the RO and OB cohorts versus the IB cohort [49].
Resistance to BTKi presents a substantial challenge in BTKi-based therapy. Suggested mechanisms of resistance are mutations at the BTK binding site [50], regulation of BCR signaling downstream of BTK via CARD11, TNFAIP3, KLHL14, and PIM1 [51], mutations in the chemokine CXCR4 receptor [52], compensatory overexpression of CD79B, overactivation of the PI3K/mTOR, p38 mitogen-activated protein kinase (MAPK) [53] and TLR/MYD88 pathways [54]. Agents that target these compensatory pathways are of interest in the treatment of PCNSL. IRAK-4, a critical downstream component of the TLR/MYD88 pathway, is one such target. Emavusertib, an IRAK-4 inhibitor, has demonstrated a 68% improvement in median survival in PCNSL xenograft models [55] and is currently under investigation in a phase 1/2 trial for R/R PCNSL (Table 3).
 Click to view | Table 3. Ongoing Clinical Trials of Targeted Agents for PCNSL |
Immunomodulatory drugs (IMiDs)
Lenalidomide and pomalidomide are second- and third-generation agents that bind cereblon (CRBN), a protein that is a component of the E3 ubiquitin ligase complex. These drugs exert pleiotropic antitumor effects via immunomodulatory, antiangiogenic, and direct apoptotic properties. The cytotoxic effects of IMiDs are mediated through the suppression of IRF4 expression with the subsequent downregulation of the pro-survival NF-kB signaling via a feedback loop in the chronically activated B cells of PCNSL [56], as well as the modulation the PI3K pathway, which results in anti-angiogenic effects [57]. IMiDs reduce the expression of the anti-apoptotic protein BCL-2, enhance natural killer (NK)-cell function, and promote T-cell proliferation and cytokine production [58]. Additionally, IMiDs influence the macrophages within the TME, with pomalidomide demonstrating the capacity to shift macrophage polarization from the pro-tumor M2 to the anti-tumor M1 phenotype and to functionally enhance the phagocytic activity of macrophages [59]. Notably, both agents have shown the ability to penetrate the BBB [41].
Lenalidomide has demonstrated clinical efficacy in R/R PCNSL; however, it has not resulted in sustained therapeutic responses [60, 61]. The phase 1 study of pomalidomide and dexamethasone in R/R PCNSL demonstrated an ORR of 48% (12 of 25 evaluable patients), CR/CRu of 32% (8/25), with a median PFS for responders of 9 months [41].
IMiD-based combination therapies for PCNSL have been investigated as well, although without significant improvement in response outcomes. Ghesquieres et al reported findings of a prospective phase 2 study evaluating the combination of lenalidomide and rituximab. The induction treatment consisted of eight 28-day cycles of rituximab 375 mg/m2 on the first day of the cycle and lenalidomide 20 mg daily, followed by a 12-month maintenance phase of lenalidomide 10 mg daily in responders. The study reported an ORR of 36% (16/45 subjects), with 13 of the responding 16 patients having achieved CR/CRu. The median PFS was 7.8 months, and the median OS was 17.7 months [42]. A case series by the French LOC network evaluated the efficacy of combination rituximab, lenalidomide, and ibrutinib in R/R PCNSL. Among the 14 patients who received this combination treatment, CR was achieved in 29% (4/14) with a median time to CR of 1.25 months. The 1-year PFS and OS rates were 40% and 53%, respectively. Although most patients did not achieve durable remissions, this therapy served as a bridge for three of the patients to receive consolidative treatment with ASCT, WBRT, and chimeric antigen receptor (CAR) T-cell therapy [62]. The clinical efficacy of IMiDs in chemotherapy-free treatment regimens continues to be investigated (Table 3).
Immune checkpoint inhibitors (ICIs)
The enrichment in chromosome 9p24.1 copy number alterations in cells of PCNSL, resulting in the increased expression of the PD-L1 and PD-L2 genes, has informed the investigation of ICIs, agents that block interactions between PD-L1/PD-L2 and their corresponding receptors, PD-1/PD-2, in the treatment of PCNSL. While promising as a potential therapy, most of the data on the efficacy of ICIs in R/R PCNSL has been derived from case series and retrospective studies, as so far only one trial (NCT02857426) reported outcomes of single-agent nivolumab (anti-PD-1 monoclonal antibody) in R/R PCNSL and primary testicular lymphoma, with an ORR of 6.4% [63].
Nayak et al reported a series of four patients with R/R PCNSL and one patient with CNS relapse of primary testicular lymphoma, all of whom received treatment with single agent nivolumab. Four out of the five patients achieved CR and one patient achieved a partial response (PR). Three out of the five patients remained progression-free at 13 - 17 months [64]. Gavrilenko et al reported a case series of nivolumab treatment of eight patients with PCNSL and one patient with primary testicular lymphoma with CNS involvement; two of the patients were previously untreated. At a median follow-up of 18 months, seven patients (78%) demonstrated an objective response, with three (33%) achieving CR [65].
Zhang et al reported preliminary results of a phase 2 study of orelabrutinib and sintilimab, a new generation anti-PD-1 monoclonal antibody. Thirteen patients were enrolled, with 10 having completed all prescribed treatment cycles while three had to discontinue due to disease progression. Four patients achieved CR, one CRu, three PR, and the estimated median 1-year PFS rate was 67.7% [43] (Tables 1 and 2). Other ICI-based strategies are under investigation, including GNC-038, a tetra-specific T-cell engager targeting CD3xCD19x4-1BBxPD-L1, currently being studied in a phase 1 trial in patients with R/R PCNSL and secondary CNS lymphoma (Table 3).
Chimeric antigen receptor (CAR) T-cell therapy
CARs are engineered synthetic receptors expressed on autologous lymphocytes, enabling them to recognize and eliminate cells that express a target antigen [66]. A CAR molecule is composed of the variable region of a high-affinity monoclonal antibody, which is connected to the intracellular signaling domain of a T-cell receptor (TCR) complex. This structure enables T-cell-mediated cytotoxicity that functions independently of MHC expression [67]. The approval of the anti-CD19 agent tisagenlecleucel (tisa-cel) by the US Food and Drug Administration (FDA) in 2017 for pediatric and young adult patients with R/R B-cell acute lymphoblastic leukemia (ALL) [68] marked a breakthrough in the treatment of hematologic malignancies. The FDA has since approved three anti-CD19 CAR T-cell therapies, axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel) and tisa-cel, for the treatment of R/R DLBCL [69-71].
The capacity of CAR T-cells to access the CNS was first observed in a clinical study of this therapy administered to pediatric patients diagnosed with R/R ALL. CAR T-cells demonstrated the ability to infiltrate and expand within the CNS. At the time of cell infusion, two patients presented with CNS leukemia which resolved following the detection of anti-CD19 CAR T-cells in the CSF [72].
Patients with CNS involvement of DLBCL were mostly excluded from prospective trials of anti-CD19 CAR T-cell therapy due to the concern for an increased risk of neurotoxicity, particularly immune-effector cell-associated neurotoxicity syndrome (ICANS), a known adverse effect associated with CAR T-cell therapy. Among the three pivotal anti-CD19 CAR T-cell trials for DLBCL, only one permitted the enrollment of patients with secondary CNS involvement of DLBCL and none of these trials were designed to prospectively investigate these agents in PCNSL. In contrast to the ZUMA-1 trial of axi-cel [73] and JULIET trial of tisa-cel [74], the TRANSCEND NHL 001 trial of liso-cel permitted the inclusion of patients with CNS involvement of systemic DLBCL. Among the 344 enrolled subjects in the TRANSCEND NHL 001 trial, seven patients had secondary CNS involvement. Two of the seven treated patients experienced neurological events, both classified as grade 3. Six patients had evaluable disease, three of whom achieved CR [70].
Most of the current body of evidence regarding the safety and efficacy of CAR T-cell therapy in PCNSL is derived from retrospective studies and small prospective trials. Although data are limited, patients with heavily pretreated PCNSL exhibit clinically significant responses to anti-CD19 CAR T cells. Frigault et al reported outcomes of a phase 1/2 trial of tisa-cel in 12 patients with relapsed PCNSL. The ORR was 58% (7/12 patients), with a CR rate of 50% (6/12). After a median follow-up period of 12 months, three patients remained in sustained remission. Five out of the 12 patients (41.6%) had low-grade ICANS, and only one patient experienced grade 3 ICANS [44] (Tables 1 and 2).
The French LOC network reported retrospective findings of a cohort of patients with R/R PCNSL treated with a single infusion of either axi-cel or tisa-cel. A total of nine patients were treated with CAR T cells (axi-cel = 2, tisa-cel = 7). Eight patients had brain parenchymal involvement, and one patient had isolated CSF disease. The best response to CAR T cells was CR in 5/9 (axi-cel = 2, tisa-cel = 3) and PR in 1/9 patients (tisa-cel). The 6-month PFS and OS rates were 44% and 89%, respectively. Among responders, the median PFS was 210 days. Seven patients experienced cytokine release syndrome (CRS), the maximum being grade 3 after tisa-cel. ICANS of any grade was observed in five patients, including one instance of grade 3 following tisa-cel administration and one instance of grade 4 following axi-cel administration [75].
The LOC network reported real-world data for 25 patients with PCNSL who had received anti-CD19 CAR T cells (tisa-cel = 16, axi-cel = 9). The best response was CR in 16 patients (64%) and PR in four patients (16%). One year following leukapheresis, the PFS rate was 43%, while the relapse-free survival (RFS) rate was 79% for patients who achieved CR or PR. The overall median OS was 21 months. Three patients experienced grade 4 neurotoxicity, with neurological deterioration persisting for more than 3 months. Notably, there was one fatal case of neurotoxicity involving a 74-year-old woman who was in CR 4 months following tisa-cel infusion. Among the other two patients with prolonged neurotoxic effects, one fully recovered 6 months after CAR T-cell infusion, while the other continued to undergo neurological recovery 9 months post-infusion [76]. While this report highlights the potential risks associated with the use of anti-CD19 CAR T cells in PCNSL, the majority of documented patient outcomes suggest that the toxicity profiles of these therapies are comparable to those observed in clinical trials of these agents for systemic DLBCL [77]. Ultimately, prospective clinical trials are essential to evaluate the safety and efficacy of anti-CD19 CAR T cells in PCNSL.
| Conclusion and Future Directions | ▴Top |
The treatment paradigm of PCNSL is at an inflection point. HD-MTX continues to be the cornerstone of initial treatment, but its use is fraught with difficulties, primarily because of its substantial systemic toxicity, which poses a considerable risk to patients with compromised organ function. This is particularly problematic as PCNSL predominantly affects older adults who are already in the most medically fragile stages of their lives. Despite undergoing treatment, a substantial proportion of patients still face the challenge of disease recurrence, and there is an absence of established therapies for relapsed disease. Despite these limitations, improved understanding of the genetic and molecular foundations of PCNSL provides opportunity to investigate rationally designed targeted therapies for PCNSL, with the goal of moving them to the upfront setting, perhaps eventually obviating the need for HD-MTX chemotherapy.
The most pressing need in identifying effective novel agents for PCNSL is in determining which formulations and dosages can successfully traverse the BBB. It is also imperative to study these agents in combinations, as a synergistic approach is essential for targeting the biological pathways of PCNSL, thereby optimizing clinical outcomes and achieving sustained responses. Furthermore, given the disease’s limited treatment options and suboptimal outcomes, it is important to explore these agents as part of the initial treatment strategy of PCNSL, rather than limiting their evaluation to relapsed cases. Clinical trials utilizing window designs may identify which patients derive the greatest benefit from combination targeted agents. This approach enables non-responders to transition to established treatments incorporating HD-MTX, while responders can continue with the investigational treatment before proceeding to consolidation.
In the context of consolidation in PCNSL, a pivotal question focuses on discerning which patients are most likely to derive substantial benefit from consolidation and identifying those who should instead be closely monitored for potential recurrence. Circulating tumor DNA (ctDNA) has emerged as a promising biomarker for detecting residual disease in systemic DLBCL [78]. ctDNA, particularly when sourced from the CSF, is under investigation as a means to improve risk and response evaluation in PCNSL, potentially having a role in informing decisions regarding the necessity of consolidation therapy [79].
While CAR T cells have demonstrated clinical efficacy in PCNSL, there is a need for prospective clinical trials to comprehensively assess the therapeutic potential of this strategy in PCNSL. The toxicity profiles of CAR T cells in PCNSL, as observed thus far, align closely with those reported in systemic DLBCL. Furthermore, the use of CAR T cells in PCNSL warrants consideration of their potential as a consolidation therapy, particularly in comparison to ASCT.
Novel agents that are currently available for systemic DLBCL, but not yet explored in PCNSL, warrant further investigation. Since a significant portion of PCNSL expresses CD79b, the anti-CD79b antibody drug conjugate, polatuzumab vedotin, may have an application in the treatment of PCNSL. The primary challenge of utilizing this agent, however, would be the limitation posed by this large protein complex to be able to penetrate the BBB. Bispecific T-cell engagers (BiTEs) represent an innovative class of T-cell redirecting antibodies that concurrently bind to tumor cell surface antigens and immune effector cells. Epcoritamab and glofitamab have demonstrated clinically significant ORR and CR rates in patients with R/R DLBCL [80, 81] and have received FDA approval for the treatment of R/R systemic DLBCL. The study of the safety and efficacy of BiTEs in PCNSL warrants further investigation in clinical trials.
The prevalence of PCNSL among older adults, who often demonstrate reduced medical fitness, sometimes impedes their participation in clinical trials. This exclusion is frequently attributed to their inability to satisfy the standard inclusion criteria established by trial investigators, criteria that have historically excluded patients with greater frailty. This highlights the necessity to reevaluate our criteria for acceptable medical fitness in a context-sensitive manner and to introduce more appropriate flexibility in trial enrollment criteria. Such flexibility should aim to accurately reflect a real-world patient demographic while ensuring the safe inclusion of patients with reasonable medical needs. This approach could expand the range of treatment options available for PCNSL within clinical trials and influence standard care practices.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
TG was responsible for the concept design of the manuscript, literature search, synthesis of the information, draft, edits and final version of the manuscript.
Data Availability
The author declares that data supporting the findings of this review are available within the manuscript.
Abbreviations
ASCT: autologous stem cell transplantation; BTK: Bruton’s tyrosine kinase; CAR: chimeric antigen receptor; CNS-DLBCL: primary diffuse large B-cell lymphoma of the CNS; CRS: cytokine release syndrome; ICANS: immune effector cell-associated neurotoxicity syndrome; ICIs: immune checkpoint inhibitors; IMiDs: immunomodulatory drugs; MYD88: myeloid differentiation primary response 88; NF-κB: nuclear factor-kappa B; NHL: non-Hodgkin lymphoma; PCNSL: primary CNS lymphoma; TME: tumor microenvironment; WBRT: whole-brain radiotherapy
| References | ▴Top |
- Green K, Munakomi S, Hogg JP. Central nervous system lymphoma. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Radke J, Ishaque N, Koll R, Gu Z, Schumann E, Sieverling L, Uhrig S, et al. The genomic and transcriptional landscape of primary central nervous system lymphoma. Nat Commun. 2022;13(1):2558.
doi pubmed - Lv C, Wang J, Zhou M, Xu JY, Chen B, Wan Y. Primary central nervous system lymphoma in the United States, 1975-2017. Ther Adv Hematol. 2022;13:20406207211066166.
doi pubmed - Yu J, Du H, Ye X, Zhang L, Xiao H. High-dose methotrexate-based regimens and post-remission consolidation for treatment of newly diagnosed primary CNS lymphoma: meta-analysis of clinical trials. Sci Rep. 2021;11(1):2125.
doi pubmed - Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296-305.
doi pubmed - Tatarczuch M, Paul E, Gilberston M, Gregory GP, Tam C, Quach H, Bazargan A, et al. Excellent outcomes in older patients with primary CNS lymphoma treated with R-MPV/cytarabine without whole brain radiotherapy or autologous stem cell transplantation therapy. Leuk Lymphoma. 2021;62(1):112-117.
doi pubmed - Henry JM, Heffner RR, Jr., Dillard SH, Earle KM, Davis RL. Primary malignant lymphomas of the central nervous system. Cancer. 1974;34(4):1293-1302.
doi pubmed - Lin CH, Yang CF, Yang HC, Fay LY, Yeh CM, Kuan AS, Wang HY, et al. Risk prediction for early mortality in patients with newly diagnosed primary CNS lymphoma. J Cancer. 2019;10(17):3958-3966.
doi pubmed - Ferreri AJM, Cwynarski K, Pulczynski E, Fox CP, Schorb E, Celico C, Falautano M, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. 2022;36(7):1870-1878.
doi pubmed - Nayak L, Hedvat C, Rosenblum MK, Abrey LE, DeAngelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525-529.
doi pubmed - Zhang Y, Zhou DB. Primary central nervous system lymphoma: status and advances in diagnosis, molecular pathogenesis, and treatment. Chin Med J (Engl). 2020;133(12):1462-1469.
doi pubmed - Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, Wang JQ, et al. A Probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell. 2020;37(4):551-568.e514.
doi pubmed - Montesinos-Rongen M, Siebert R, Deckert M. Primary lymphoma of the central nervous system: just DLBCL or not? Blood. 2009;113(1):7-10.
doi pubmed - Phelan JD, Young RM, Webster DE, Roulland S, Wright GW, Kasbekar M, Shaffer AL, 3rd, et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature. 2018;560(7718):387-391.
doi pubmed - Montesinos-Rongen M, Godlewska E, Brunn A, Wiestler OD, Siebert R, Deckert M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011;122(6):791-792.
doi pubmed - Vermaat JS, Somers SF, de Wreede LC, Kraan W, de Groen RAL, Schrader AMR, Kerver ED, et al. MYD88 mutations identify a molecular subgroup of diffuse large B-cell lymphoma with an unfavorable prognosis. Haematologica. 2020;105(2):424-434.
doi pubmed - Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11-19.
doi pubmed - Young RM, Phelan JD, Wilson WH, Staudt LM. Pathogenic B-cell receptor signaling in lymphoid malignancies: New insights to improve treatment. Immunol Rev. 2019;291(1):190-213.
doi pubmed - Thompsett AR, Ellison DW, Stevenson FK, Zhu D. V(H) gene sequences from primary central nervous system lymphomas indicate derivation from highly mutated germinal center B cells with ongoing mutational activity. Blood. 1999;94(5):1738-1746.
pubmed - Montesinos-Rongen M, Purschke FG, Brunn A, May C, Nordhoff E, Marcus K, Deckert M. Primary Central Nervous System (CNS) lymphoma B cell receptors recognize CNS proteins. J Immunol. 2015;195(3):1312-1319.
doi pubmed - Thurner L, Preuss KD, Bewarder M, Kemele M, Fadle N, Regitz E, Altmeyer S, et al. Hyper-N-glycosylated SAMD14 and neurabin-I as driver autoantigens of primary central nervous system lymphoma. Blood. 2018;132(26):2744-2753.
doi pubmed - Montauti E, Oh DY, Fong L. CD4(+) T cells in antitumor immunity. Trends Cancer. 2024;10(10):969-985.
doi pubmed - Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, Dunford AJ, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869-881.
doi pubmed - Sasayama T, Tanaka K, Mizowaki T, Nagashima H, Nakamizo S, Tanaka H, Nishihara M, et al. Tumor-associated macrophages associate with cerebrospinal fluid interleukin-10 and survival in primary central nervous system lymphoma (PCNSL). Brain Pathol. 2016;26(4):479-487.
doi pubmed - Furuse M, Kuwabara H, Ikeda N, Hattori Y, Ichikawa T, Kagawa N, Kikuta K, et al. PD-L1 and PD-L2 expression in the tumor microenvironment including peritumoral tissue in primary central nervous system lymphoma. BMC Cancer. 2020;20(1):277.
doi pubmed - Jin Q, Jiang H, Han Y, Zhang L, Li C, Zhang Y, Chai Y, et al. Tumor microenvironment in primary central nervous system lymphoma (PCNSL). Cancer Biol Ther. 2024;25(1):2425131.
doi pubmed - Calimeri T, Steffanoni S, Gagliardi F, Chiara A, Ferreri AJM. How we treat primary central nervous system lymphoma. ESMO Open. 2021;6(4):100213.
doi pubmed - Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217-227.
doi pubmed - Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, Cheson BD, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061-3068.
doi pubmed - Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403-1410.
doi pubmed - Pescovitz MD. Rituximab, an anti-cd20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6(5 Pt 1):859-866.
doi pubmed - Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, Ignoffo R, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466-468.
doi pubmed - Birnbaum T, Stadler EA, von Baumgarten L, Straube A. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. J Neurooncol. 2012;109(2):285-291.
doi pubmed - Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, Grossman SA, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83(3):235-239.
doi pubmed - Gregory G, Arumugaswamy A, Leung T, Chan KL, Abikhair M, Tam C, Bajel A, et al. Rituximab is associated with improved survival for aggressive B cell CNS lymphoma. Neuro Oncol. 2013;15(8):1068-1073.
doi pubmed - Bromberg JEC, Issa S, Bakunina K, Minnema MC, Seute T, Durian M, Cull G, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019;20(2):216-228.
doi pubmed - Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, Yang Y, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833-843.e835.
doi pubmed - Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D, Codega P, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018-1029.
doi pubmed - Soussain C, Choquet S, Blonski M, Leclercq D, Houillier C, Rezai K, Bijou F, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121-130.
doi pubmed - Narita Y, Nagane M, Mishima K, Terui Y, Arakawa Y, Yonezawa H, Asai K, et al. Phase I/II study of tirabrutinib, a second-generation Bruton's tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma. Neuro Oncol. 2021;23(1):122-133.
doi pubmed - Tun HW, Johnston PB, DeAngelis LM, Atherton PJ, Pederson LD, Koenig PA, Reeder CB, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018;132(21):2240-2248.
doi pubmed - Ghesquieres H, Chevrier M, Laadhari M, Chinot O, Choquet S, Molucon-Chabrot C, Beauchesne P, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective 'proof of concept' phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA)dagger. Ann Oncol. 2019;30(4):621-628.
doi pubmed - Zhang Y, Wang W, Zhao D, Zhang Y, Zhang W, Zhou D. S224: preliminary results of a phase II study of orelabrutinib in combination with anti-PD-1 monoclonal antibody in refractory or relapsed primary CNS lymphoma. HemaSphere. 2022;6:125-126.
doi - Frigault MJ, Dietrich J, Gallagher K, Roschewski M, Jordan JT, Forst D, Plotkin SR, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022;139(15):2306-2315.
doi pubmed - Samhouri Y, Ali MM, Jayakrishnan T, et al. Autologous stem cell transplantation (ASCT) versus whole brain radiation (WBRT) as a consolidation therapy in primary CNS lymphoma (PCNSL): a nationwide analysis. Journal of Clinical Oncology. 2021;39(15).
doi - Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, Grimm S, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971-3979.
doi pubmed - Strati P, De Vos S, Ruan J, Maddocks KJ, Flowers CR, Rule S, Patel P, et al. Acalabrutinib for treatment of diffuse large B-cell lymphoma: results from a phase Ib study. Haematologica. 2021;106(10):2774-2778.
doi pubmed - Wu JJ, Wang WH, Dong M, Ma SS, Zhang XD, Zhu LN, Niu ST, et al. Orelabrutinib-bruton tyrosine kinase inhibitor-based regimens in the treatment of central nervous system lymphoma: a retrospective study. Invest New Drugs. 2022;40(3):650-659.
doi pubmed - Qiao L, Liu Q, Huang C. Orelabrutinib versus ibrutinib for patients with refractory/relapsed primary central nervous system lymphoma: An efficacy and safety analysis. Medicine (Baltimore). 2023;102(27):e33880.
doi pubmed - Epperla N, Shana'ah AY, Jones D, Christian BA, Ayyappan S, Maddocks K, Woyach JA. Resistance mechanism for ibrutinib in marginal zone lymphoma. Blood Adv. 2019;3(4):500-502.
doi pubmed - Nakhoda S, Vistarop A, Wang YL. Resistance to Bruton tyrosine kinase inhibition in chronic lymphocytic leukaemia and non-Hodgkin lymphoma. Br J Haematol. 2023;200(2):137-149.
doi pubmed - Cao Y, Hunter ZR, Liu X, Xu L, Yang G, Chen J, Patterson CJ, et al. The WHIM-like CXCR4(S338X) somatic mutation activates AKT and ERK, and promotes resistance to ibrutinib and other agents used in the treatment of Waldenstrom's Macroglobulinemia. Leukemia. 2015;29(1):169-176.
doi pubmed - Kim JH, Kim WS, Ryu K, Kim SJ, Park C. CD79B limits response of diffuse large B cell lymphoma to ibrutinib. Leuk Lymphoma. 2016;57(6):1413-1422.
doi pubmed - Wang H, Zhang W, Yang J, Zhou K. The resistance mechanisms and treatment strategies of BTK inhibitors in B-cell lymphoma. Hematol Oncol. 2021;39(5):605-615.
doi pubmed - Von Roemeling CA, Doonan BP, Klippel K, Schultz D, Hoang-Minh L, Trivedi V, Li C, et al. Oral IRAK-4 inhibitor CA-4948 is blood-brain barrier penetrant and has single-agent activity against CNS lymphoma and melanoma brain metastases. Clin Cancer Res. 2023;29(9):1751-1762.
doi pubmed - Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R. Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol. 2013;160(4):487-502.
doi pubmed - Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, Shirley MA, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1-2):56-63.
doi pubmed - Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, Wallace P, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140(1):36-45.
doi pubmed - Li Z, Qiu Y, Personett D, Huang P, Edenfield B, Katz J, Babusis D, et al. Pomalidomide shows significant therapeutic activity against CNS lymphoma with a major impact on the tumor microenvironment in murine models. PLoS One. 2013;8(8):e71754.
doi pubmed - Rubenstein JL, Geng H, Fraser EJ, Formaker P, Chen L, Sharma J, Killea P, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595-1607.
doi pubmed - Molaie D, Hu J, Rudnick J. Lenalidomide as treatment for relapsed or refractory primary CNS lymphoma: a single institutional experience. Neuro-Oncology. 2018;20:239.
- Houillier C, Chabrot CM, Moles-Moreau MP, Willems L, Ahle G, Waultier-Rascalou A, Fornecker LM, et al. Rituximab-lenalidomide-ibrutinib combination for relapsed/refractory primary CNS lymphoma: a case series of the LOC network. Neurology. 2021;97(13):628-631.
doi pubmed - Lin N, Song Y, Zhu J. Immune checkpoint inhibitors in malignant lymphoma: Advances and perspectives. Chin J Cancer Res. 2020;32(3):303-318.
doi pubmed - Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B, Armand P, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071-3073.
doi pubmed - Gavrilenko AN, Volkov NP, Shmidt DI, et al. Nivolumab in primary CNS lymphoma and primary testicular lymphoma with CNS involvement: single center experience. Blood. 2020;136.
doi - Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
doi pubmed - Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology. 2019;8(5):e1049.
doi pubmed - O'Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, Gavin D, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019;25(4):1142-1146.
doi pubmed - Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654.
doi pubmed - Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839-852.
doi pubmed - Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, Kato K, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629-639.
doi pubmed - Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517-528.
doi pubmed - Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544.
doi pubmed - Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jager U, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56.
doi pubmed - Alcantara M, Houillier C, Blonski M, Rubio MT, Willems L, Rascalou AW, Le Garff-Tavernier M, et al. CAR T-cell therapy in primary central nervous system lymphoma: the clinical experience of the French LOC network. Blood. 2022;139(5):792-796.
doi pubmed - Choquet S, Soussain C, Azar N, Morel V, Metz C, Ursu R, Waultier-Rascalou A, et al. CAR T-cell therapy induces a high rate of prolonged remission in relapsed primary CNS lymphoma: Real-life results of the LOC network. Am J Hematol. 2024;99(7):1240-1249.
doi pubmed - Cook MR, Dorris CS, Makambi KH, Luo Y, Munshi PN, Donato M, Rowley S, et al. Toxicity and efficacy of CAR T-cell therapy in primary and secondary CNS lymphoma: a meta-analysis of 128 patients. Blood Adv. 2023;7(1):32-39.
doi pubmed - Vimalathas G, Hansen MH, Cedile OML, Thomassen M, Moller MB, Dahlmann SK, Kjeldsen MLG, et al. Monitoring ctDNA in aggressive B-cell lymphoma: a prospective correlative study of ctDNA kinetics and PET-CT metrics. Blood Adv. 2025.
doi pubmed - Navrkalova V, Mareckova A, Hricko S, Hrabcakova V, Radova L, Kubes V, Porc J, et al. Reliable detection of CNS lymphoma-derived circulating tumor DNA in cerebrospinal fluid using multi-biomarker NGS profiling: insights from a real-world study. Biomark Res. 2025;13(1):71.
doi pubmed - Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, Do YR, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41(12):2238-2247.
doi pubmed - Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, Khan C, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387(24):2220-2231.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.