| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Review
Volume 000, Number 000, May 2025, pages 000-000
Evans Syndrome and COVID-19 Infection or Vaccination: A Systematic Review of Case Reports
Andrew Yacouba, e, Michael Atallaa, Anis Hasnaouib, c, Prakash V.A.K. Ramdassa, d
aSt. George’s University School of Medicine, True Blue Campus, St. George, Grenada
bFaculty of Medicine of Tunis, Tunis El Manar University, Tunis, Tunisia
cDepartment of General Surgery, Menzel Bourguiba Hospital, 1006 Tunis, Tunisia
dDepartment of Public Health and Preventive Medicine, St. George’s University School of Medicine, True Blue Campus, St. George, Grenada
eCorresponding Author: Andrew Yacoub, St. George’s University School of Medicine, True Blue Campus, St. George, Grenada
Manuscript submitted March 14, 2025, accepted April 30, 2025, published online May 13, 2025
Short title: ES Secondary to COVID-19 Infection or Vaccination
doi: https://doi.org/10.14740/jh2058
| Abstract | ▴Top |
Evans syndrome (ES) is an autoimmune disorder of unknown etiology characterized by autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP). In this systematic review, we analyzed the reported cases of ES secondary to coronavirus disease 2019 (COVID-19) infection or COVID-19 vaccination. We examined their clinical presentation, temporality between events, diagnostics tests, and treatment regimens. Our search in four databases from December 2019 to September 2023 yielded 16 case reports that met eligibility criteria for inclusion. COVID-19 and ES symptoms were defined to assess the timeline between infection/vaccination and ES onset. Finally, treatment efficacy was categorized as complete, partial, or no response based on standard hematological criteria. Eleven cases of ES were associated with COVID-19 infection, and five cases of ES were associated with COVID-19 vaccination. All 16 cases presented with anemia, thrombocytopenia, and a positive Coombs test. Four of the five patients from the vaccination subset were found to have an additional autoimmune disease as a comorbidity on presentation. For cases of ES secondary to COVID-19 infection, six patients had concomitant symptoms of COVID-19 and ES on presentation, and four patients had ES symptoms occurring from 5 days to 3 weeks following COVID-19 infection. The remaining case presented a patient with a 3-week history of ES symptoms before a positive COVID-19 test and further ES workup on admission. For the five cases of ES post-COVID-19 vaccination, all five patients presented with ES with a mean presentation time of 9 days following vaccination. Regarding treatment, intravenous immunoglobulin (IVIG) emerged as the primary regimen, administered in 13 out of the 16 cases. Among the infection-related cases, the most frequent treatment outcome was a partial response in both AIHA and ITP, observed in five of the 11 patients. In the vaccination-related cases, a partial response for AIHA and a complete response for ITP were noted in three of the five patients. Overall, while the evidence points to a temporal association especially between COVID-19 vaccination and the onset of ES, larger studies are necessary to strengthen these findings. In terms of management, early initiation of corticosteroids and IVIG appears effective as first-line therapies; however, standardized treatment protocols are needed to help reduce complications associated with COVID-19-related ES.
Keywords: Evans syndrome; COVID-19; Vaccination; Hemolytic anemia; Thrombocytopenia
| Introduction | ▴Top |
The pathogenesis of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) revealed its propensity to compromise immune responses, characterized by cytokine storms and autoimmunity [1, 2]. Numerous studies have identified a heightened risk of autoimmune diseases, including rheumatoid arthritis and systemic lupus erythematosus (SLE), following coronavirus disease 2019 (COVID-19) infection [3, 4]. In addition, studies have reported a broad range of adverse outcomes following immunization against COVID-19, ranging from immune thrombocytopenia (ITP) to myocarditis [5]. This newfound knowledge underscores the importance of thoroughly examining the intricate interplay between COVID-19 infection, COVID-19 vaccination, and the immune system.
Early observations from case reports have investigated the relationship between COVID-19 and Evans syndrome (ES), a rare autoimmune condition of unknown etiology [1, 6]. ES is characterized by the simultaneous or sequential manifestation of autoimmune hemolytic anemia (AIHA) and ITP, with less common instances of autoimmune neutropenia occurring in approximately 25% of cases [2, 7]. Since being first described in children by Robert Evans in 1951, ES has been associated with a spectrum of autoimmune and lymphoproliferative disorders in both children and adults, with an incidence of roughly 1 in 2.7 million and a slight predominance in females [8-12]. Various studies have reported a median age of diagnosis ranging from 50 to 58.5 years [10, 11, 13].
Distinguishing between primary (idiopathic) and secondary ES is vital for diagnostics and treatment, with ES usually emerging as a diagnosis of exclusion [1, 14]. The diagnostic workup for ES typically begins with a thorough history and physical examination, followed by confirmation through blood work showing a decreased platelet count (< 150 × 109/L), decreased hemoglobin (less than 13.5 g/dL for males and 12 g/dL for females), and a positive direct antiglobulin test (Coombs test) in the setting of active hemolysis [1]. First-line treatment regimens typically include corticosteroids or intravenous immunoglobulin (IVIG), and symptomatic management may require transfusions [6].
An established relationship exists between COVID-19 infection/vaccination and hematological disorders, such as ITP, thrombosis with thrombocytopenia syndrome (TTS), arterial and venous thromboembolism, and even cases of ES [15-17]. Although cases of ES secondary to COVID-19 infection or COVID-19 vaccination are well documented in the literature separately, only one systematic review presenting 12 case reports was published regarding both precipitating events, calling for a more comprehensive review of the literature. Thus, our systematic review aimed to provide an analysis of case reports on the clinical presentation, temporality, diagnostics, and treatment regimens of patients with new-onset ES secondary to COVID-19 infection or vaccination.
| Methods | ▴Top |
Study design and search strategy
A systematic review was conducted to consolidate case reports highlighting ES secondary to COVID-19 infection or COVID-19 vaccination. The literature search strategy was framed according to PRISMA guidelines and aimed to select articles published between December 2019 and September 14, 2023 [18]. An extensive search was conducted in PubMed, Embase, Google Scholar, and Scopus databases, utilizing the keywords “COVID-19” OR “SARS-CoV-2” AND “Evans Syndrome” OR “Evan’s Syndrome,” combined using the Boolean operators “AND” and “OR.” Additional case reports were identified through citation searching, bringing the total to 142 articles. The Zotero software 6.0.28 was used to organize and eliminate duplicates.
Study selection
The study selection process was carried out independently by two reviewers (AY and MA), with a third reviewer (PR) intervening in cases of disagreement. The risk of bias in each report was assessed using the Newcastle-Ottawa scale [19]. The initial screening stage excluded 20 articles based on their titles and abstracts, narrowing the selection to 57. At this stage, we eliminated non-case reports, animal studies, papers without full-text access, and those in languages other than English, Arabic, or French. Although introducing some bias, limiting the languages to English, Arabic, and French was necessary due to the authors’ language proficiency. There was no need to contact study authors for missing data. In the second stage, we screened the full texts and excluded cases with a prior diagnosis of ES before COVID-19 infection or vaccination, a history of ES relapse (patients not in complete remission), and cases where insufficient data on ES were available. We also excluded case reports in which the same patient was exposed to both COVID-19 infection and vaccination.
Data extraction
Four tables were created to illustrate the data extracted from the reports. Tables 1 and 2 [20-35] present the patients’ baseline characteristics, and Tables 3 and 4 [20-35] show the attributes that ultimately enabled us to fulfil the study’s aim. Data extraction was performed by one investigator (AY) before being reviewed by all three investigators (AY, MA, and PR). The hematological parameters of interest extracted from each case report were those used in a review by Fazeli et al [1]. This approach allowed for the utilization of relevant laboratory investigations for ES alongside a full panel of parameters.
 Click to view | Table 1. Baseline Characteristics of Patients From Case Reports Diagnosed With ES Following Infection With COVID-19 |
 Click to view | Table 2. Baseline Characteristics of Patients From Case Reports Diagnosed With ES Following COVID-19 Vaccination |
 Click to view | Table 3. Clinical Characteristics From Cases of ES Secondary to COVID-19 Infection |
 Click to view | Table 4. Clinical Characteristics of COVID-19 Vaccination Cases Associated With ES |
Temporality analysis
A distinction between the signs and symptoms of COVID-19 and ES was necessary to assess the temporality criterion between COVID-19 infection and ES presentation. The signs and symptoms used for COVID-19 included fever, chills, cough, shortness of breath, difficulty breathing, fatigue, muscle or body aches, loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea [36]. For ES, the signs and symptoms included pallor, jaundice, dizziness, shortness of breath, splenomegaly, easy bruising, bleeding, petechiae, and purpura [6]. To establish a precise timeline, we used either a confirmatory positive COVID-19 test or positive symptoms with high suspicion of COVID-19 to correlate with the patient’s initial signs and symptoms of ES. The selection depended on which variable was provided in the case report. For cases related to COVID-19 vaccination, the timeline was set between the time of vaccination and the initial presentation of ES.
Response to treatment
A similar framework was used in a survey analyzing ES in 68 cases to assess treatment efficacy in the included case reports [13]. For AIHA, a complete response to treatment was defined by a hemoglobin level of 12 g/dL or more in the absence of any transfusion or indication of hemolysis (positive direct Coombs test, elevated lactic dehydrogenase (LDH), indirect bilirubin, or reticulocytosis). A partial response was defined by a minimum hemoglobin level of 10 g/dL or at least a 2 g increase from the pre-treatment count. For thrombocytopenia, a complete response was defined as a platelet count greater than 150 × 109/L without transfusion, while a partial response was defined as a platelet count greater than 50 × 109/L or at least a two-fold increase from the pre-treatment count. If patient values from a case report did not meet the criteria outlined, the treatment was labeled as having “no response.”
| Results | ▴Top |
The primary search yielded the following results: PubMed (25), Embase (47), Google Scholar (19), Scopus (51), and citation searching (4), totaling 146 articles. After removing 65 duplicates, screening was conducted on 81 titles and abstracts (77 plus 4), followed by screening of 60 full-text articles. As a result, our systematic review included a total of 16 case reports of patients presenting with a confirmed diagnosis of ES secondary to either COVID-19 infection or COVID-19 vaccination. Eleven cases were associated with COVID-19 infection (69%) [20-30]. The other five cases were linked to COVID-19 vaccination (31%) [31-35]. A flow diagram of our search is shown in Figure 1.
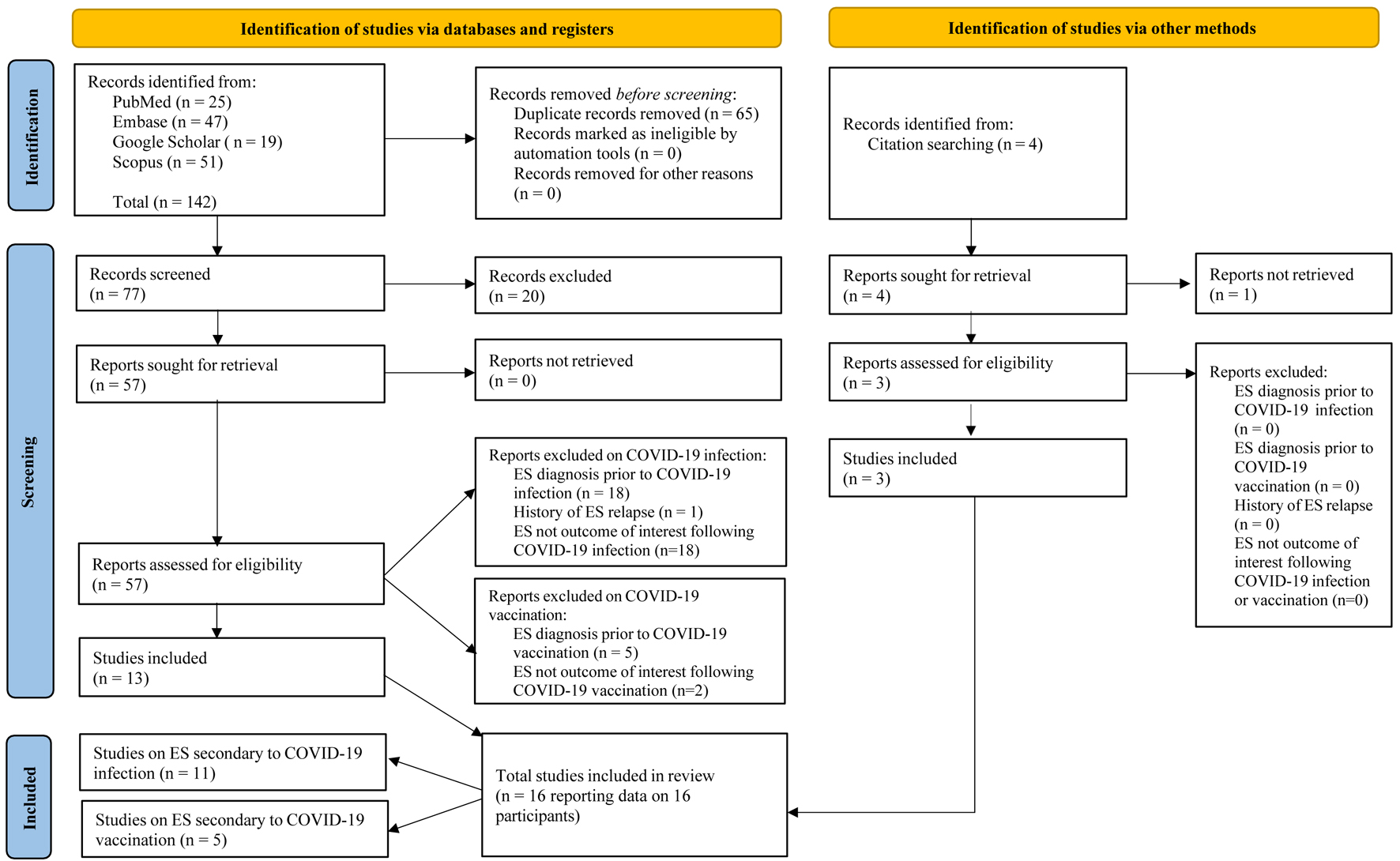 Click for large image | Figure 1. Flow chart of study selection process according to PRISMA guidelines. Only case reports were included in the systematic review. A total of 16 cases were found: 13 via databases and three via citation searching. Of the 16 case reports which were identified, 11 covered ES following COVID-19 infection, and the remaining five following COVID-19 vaccination. COVID-19: coronavirus disease 2019. |
Clinical presentation and temporality
Those with COVID-19 infection had a median age of 30 years, with six of them being male. In contrast, those with COVID-19 vaccination had a median age of 53 years, with two of them being male. Overall, eight of the patients were male (50%), with a median age of 41 years.
In the 11 cases associated with COVID-19 infection, three patients sought medical attention with initial symptoms of COVID-19 infection only, two patients with initial symptoms of ES only, and the remaining six patients with symptoms of both COVID-19 and ES occurring concurrently [20-30].
Various comorbidities and past medical histories were noted among both patient subsets. In the COVID-19 vaccination subset, an additional autoimmune disease was present in four of the five patients (80%) [31-34]. The first patient had a 2-year remission period of SLE; the second patient was diagnosed with both SLE and ES concurrently following COVID-19 vaccination; the third had localized scleroderma, also diagnosed concurrently with ES; and the fourth case featured a patient in complete remission of ES following a splenectomy in 2017 [31-34]. The baseline characteristics of the cases are presented in Tables 1 and 2. Also in this subset, four patients received the BNT162b2 (Pfizer-BioNTech) vaccine, and one patient received the ChAdOx1 nCoV-19 (AstraZeneca) vaccine [31-34].
Regarding temporality, four of the 11 cases developed ES following a COVID-19 infection [20, 22, 23, 25]. Of these four cases, two revealed time intervals of 7 and 9 days between the initial symptoms of COVID-19 and the earliest signs or symptoms of ES [20, 23]. The remaining two cases had time intervals of 5 days and 3 weeks between a positive COVID-19 test and the initial ES presentation [22, 25]. In six other cases among the 11, symptoms of COVID-19 and ES presented concomitantly, resulting in both a positive COVID-19 test and a clinical diagnosis of ES upon admission [21, 24, 26-29]. Lastly, a patient with a 3-week onset of ES symptoms was diagnosed with ES secondary to immune destruction when their COVID-19 test returned a positive result on the day of admission [30].
In each of the five cases investigating ES and COVID-19 vaccination, signs and symptoms of anemia and thrombocytopenia were present in all patients upon admission [31-35]. Cutaneous manifestations, including petechiae, purpura, ecchymosis, epistaxis, or bleeding, were noted in all five cases, indicating signs of thrombocytopenia. Icterus or pallor was also present in all five reports, possibly indicating anemia. Regarding the temporality of events, all ES presentations occurred following COVID-19 vaccination. The time between vaccination and the onset of ES signs was explicitly reported in four of the five cases with a mean time of 9 days (standard deviation (SD) 5.1).
Diagnostics
In all 16 cases of ES secondary to COVID-19 infection, anemia and thrombocytopenia were present without neutropenia [20-30]. The median hemoglobin and platelet count that led to the diagnosis of ES in patients from the 11 reports associated with COVID-19 infection were 6.2 g/dL (SD 2.2) and 37 × 109/L (SD 46), respectively. Hemoglobin levels (regardless of sex) and platelet counts were decreased in each case, ranging from 2.5 to 10 g/dL for hemoglobin and from 2 × 109/L to 117 × 109/L for platelet count. A positive direct Coombs test (DAT) was recorded in each of the 11 cases [20-30]. The median LDH level from the eight cases that included this test was 792 U/L, with a range from 425 to 1,953 U/L [21, 23, 25, 27-31]. The reticulocyte ratio was included in seven cases with the following values: 0.61%, 1.9%, 3.65%, 7%, 13.73%, 22%, and 36% [20, 23-26, 29, 30]. Five cases included indirect bilirubin levels, with a range from 2.9 to 36.24 mg/dL [24-27, 29]. Spherocytes were observed on a peripheral blood smear in five of the cases, with an additional two cases showing anisopoikilocytosis without spherocytes [23, 25-30].
For the five cases of ES secondary to COVID-19 vaccination, the median hemoglobin and platelet counts were 6.9 g/dL (SD 2.5) and 8 × 109/L (SD 16), respectively [31-35]. Hemoglobin and platelet ranges were 4.5 to 10 g/dL for hemoglobin and 1 × 109/L to 39 × 109/L for platelets [31-35]. A direct Coombs test was positive in all five cases (100%) [31-35]. LDH levels were recorded in each case, with a median of 633 U/L and a range from 248 to 2,226 U/L [31-35]. Indirect bilirubin was assessed in three cases, ranging from 1.2 to 6.1 mg/dL [31, 32, 35]. A blood smear was carried out in only one case, revealing mild anisocytosis [33].
In case reports that listed differentials for the diagnosis of ES, HELLP syndrome, atypical hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura were all rightfully excluded in a patient at 39 weeks’ gestation due to the absence of schistocytes on the blood smear [25]. In another case, viral serologies (hepatitis B virus, hepatitis C virus, and human immunodeficiency virus), a blood culture, an anti-nuclear antibodies test, and a rheumatoid factor test were all investigated before suspecting ES [29]. A third case ruled out thrombotic thrombocytopenic purpura via flow cytometry [22]. All three of these cases were from the COVID-19 infection subset. Additionally, a bone marrow biopsy was conducted in three cases: two from ES cases following COVID-19 infection and one from the COVID-19 vaccination subset [22, 24, 32].
Treatment regimens
For the COVID-19 infection subset, the most common therapies administered were IVIG and blood cell transfusions (platelets, red blood cells (RBCs), and/or plasma), given in eight and six of the 11 case reports, respectively [20-30]. Only one case did not include systemic glucocorticoids as part of their treatment regimen [23]. That patient went on to survive. In the five cases of ES secondary to COVID-19 vaccination, IVIG was used in four patients [32-35]. Also, at least one glucocorticoid was administered in each case [31-35].
For treatment responses in cases of COVID-19 infection, the treatment of AIHA resulted in zero complete responses, five partial responses, five no responses, and one inconclusive response due to lack of data specificity. Meanwhile, treatment of ITP yielded one complete response, eight partial responses, one no response, and one inconclusive response also due to lack of specificity. While comparing each of the 11 cases with regards to treatment response, the most frequent combination was a partial response for both AIHA and ITP, observed in five of the 11 cases [24-26, 28, 29].
For the five cases regarding COVID-19 vaccination, the treatment response for the AIHA component of ES included zero complete responses, four partial responses, and one no response. For ITP, there were three complete responses and two partial responses. The most frequent combination was a partial response for AIHA and a complete response for ITP, seen in three of the five cases [32, 34, 35].
To provide insight into the severity of SARS-CoV-2 in the case reports, oxygen supplementation was administered to five of the 11 patients infected with COVID-19 due to decreased oxygen saturation [21, 23, 25-27].
Following their diagnosis of ES, 15 of the 16 patients made a full recovery. The remaining patient died from intracerebral hemorrhage [30]. This patient was from the COVID-19 infection subset.
After analyzing treatment responses by age and sex within the COVID-19 infection subset, the most favorable outcome - a partial response to both AIHA and ITP - was observed in two males and two females, all under the age of 30. The least favorable response was noted in a 33-year-old male who showed no improvement in either AIHA or ITP and died on the third day of hospitalization.
In contrast, this age-related trend was not seen in the vaccination subset. In this group, the most favorable response, defined as a partial response to AIHA and a complete response to ITP, was observed in two males and one female, aged 85, 47, and 56, respectively.
Regarding treatment responses based on the presence or absence of pre-existing autoimmune diseases, one case in the COVID-19 vaccine subset involved a patient without any autoimmune comorbidities at presentation aside from ES. This patient demonstrated a partial response to AIHA and a complete response to ITP, mirroring the treatment response seen in two other cases within the subset but achieving a more favorable overall outcome.
| Discussion | ▴Top |
In this systematic review, we analyzed 11 reported cases of ES secondary to COVID-19 infection and five reported cases of ES following COVID-19 vaccination. We analyzed the patient’s clinical presentation leading up to admission, the temporality measure between the patient’s COVID-19 infection/vaccination and ES diagnosis, and finally, their progression regarding diagnosis and treatment regimens.
Clinical presentation and temporality
The calculated median ages and sex ratio align well with established knowledge that ES has a higher incidence in the fifth decade of life, with a slight preponderance in females [10-12, 37]. In the COVID-19 infection subset, data on pre-existing comorbidities were heterogeneous and varied from case to case without clear trends. However, marked patterns emerged in the COVID-19 vaccination subset. Notably, four of the five patients had a history of an autoimmune disorder or developed another autoimmune disorder upon admission in addition to ES following COVID-19 vaccination [31-34]. In two of those four cases, ES was reported alongside SLE, an association that is well documented in the literature [31, 34]. While the mechanism behind their co-occurrence is not fully understood, it is theorized that cross-reactions between phospholipids and cardiolipin in the presence of cell damage may explain why they can present concurrently in the same patient [38]. Furthermore, an analysis of 68 adult cases of ES found that autoimmune disorders, particularly SLE, are the most common conditions to present with ES [13]. A potential interaction with other autoimmune disorders could, therefore, be contributing to the pathogenesis of ES. Given that four out of five patients in the vaccine subset presented with an additional autoimmune disease beyond ES, it is essential to thoroughly screen patients with suspected ES for other underlying autoimmune conditions. A comprehensive evaluation may help clinicians tailor more effective management strategies according to the severity and nature of the coexisting autoimmune disorder. One study identified alopecia totalis, Behcet’s disease, Crohn’s disease, and bullous pemphigoid as the most common autoimmune conditions following COVID-19, followed by alopecia areata, vitiligo, ulcerative colitis, rheumatoid arthritis, SLE, Sjogren’s syndrome, and ankylosing spondylitis [39]. Therefore, it is advisable to conduct a diagnostic workup, including laboratory testing for these conditions, in patients with suspected ES.
Although there remains uncertainty regarding the adverse effects of COVID-19 immunization, there is increasing evidence of distinguishable impacts based on the vaccine profile. Many studies have shown a higher risk of Bell’s palsy and myocarditis with the BNT162b2 (mRNA-based) vaccine compared to the ChAdOx1 vaccine (viral vector-based) [40]. In contrast, the ChAdOx1 vaccine has been shown to be associated with Guillain-Barre syndrome and thrombotic thrombocytopenia [40]. In light of these findings, clinicians need to weigh the risks of the adverse effect profile of each vaccine against the benefits of vaccination, which is particularly crucial for autoimmune patients given their higher risk of COVID-19 compared to the general population [41]. Since both types of vaccines (AstraZeneca and Pfizer) have been linked to autoimmune disorders in the literature, including ES as observed in the five cases reviewed here, additional case reporting is also necessary to allow for a more comprehensive evaluation of vaccine safety.
Regarding the temporal relationship between COVID-19 infection and ES, we established that as long as ES was not clinically diagnosed before the patient was exposed to COVID-19, a temporal association cannot be ruled out. This premise was important to establish, especially in case reports where symptoms of COVID-19 and ES coincided in the patient, as seen in six of the 11 cases following COVID-19 infection. This made it difficult to determine which event occurred first. Providing such leniency also accounted for possible asymptomatic cases of ES. In our analysis, two patients were asymptomatic for ES until they presented with symptoms of COVID-19, which then prompted further hematological workup [22, 25]. Although evidence of asymptomatic ES cases is scarce in the literature, asymptomatic cases of independent ITP or AIHA are more common [42, 43]. With an incubation period of up to 2 weeks, COVID-19 can also be asymptomatic [44]. This was the case in one patient in this review, who did not exhibit any major symptoms of COVID-19 leading up to the diagnosis of ES [22].
A deeper understanding of the molecular mechanisms is essential for evaluating the temporality criterion in the association between COVID-19 and ES. In the case of AIHA, research suggests that the SARS-CoV-2 surface spike glycoprotein shares structural similarity with ankyrin-1, a membrane protein found on RBCs, potentially triggering a cross-reactive immune response via molecular mimicry and resulting in RBC destruction by autoantibodies [45]. Other proposed mechanisms for AIHA in the setting of COVID-19 include the exposure of hidden epitopes and the formation of neo-antigens [46, 47]. For ITP, possible explanations include the production of antibodies targeting platelet glycoproteins such as GP IIb/IIIa, GPIb/IX, or GP-V, as well as enhanced hepatic clearance of platelets and increased platelet aggregation and consumption [48].
This immune-mediated pathophysiology is not unique to COVID-19; several other viral infections have similarly been implicated in triggering ES. Notably, viruses such as Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, hepatitis C virus, and varicella-zoster virus are believed to induce ES through mechanisms like immune system dysregulation, molecular mimicry, epitope spreading, and neoantigen formation, ultimately leading to autoantibody-mediated destruction of blood cells [46]. Although the exact pathogenesis remains incompletely understood, the association between viral infections and the development of ES is well documented [46]. Similarly, these same mechanisms of immune response have been implicated in vaccine-induced ES, with reports linking vaccines such as the influenza vaccine and the hepatitis B vaccine to the development of the condition [47, 48]. However, given the rarity of these occurrences, establishing a definitive causal relationship remains challenging.
As mentioned, recent studies have highlighted neo-antigen formation as a potential mechanism contributing to autoimmune phenomena following COVID-19 infection. During SARS-CoV-2 infection, viral proteases such as NSP3 and NSP5 can cleave numerous human proteins, potentially generating novel antigenic determinants that the immune system may misidentify as foreign, leading to autoantibody production and tissue-specific damage [49]. In the context of ES, this process could promote the development of autoantibodies targeting RBCs and platelets, exacerbating cytopenias.
Diagnostics
Analysis of the 16 cases provides strong evidence that the evaluated hematological parameters are characteristic of ES. All patients demonstrated decreased hemoglobin levels and platelet counts at the time of diagnosis. Additionally, every case had a positive Coombs test, confirming the presence of AIHA. LDH and indirect bilirubin levels were consistently elevated in the cases where these markers were assessed, showing notable uniformity. However, hemolysis indicators, particularly reticulocyte counts, were not reported frequently enough to establish clear patterns or draw definitive conclusions.
In ES, the direct Coombs test result is almost invariably positive for immunoglobulin G (IgG), complement, or both [50]. Therefore, not conducting the test could compromise the accuracy of the diagnosis. The fact that each case from both subsets recorded a positive result for this test ensures high diagnostic accuracy.
None of the 16 cases investigated antiplatelet antibodies in their diagnostic approach for the ITP component of ES. Although the detection of antiplatelet antibodies seems coherent with a diagnosis of ES-associated ITP, it is not routinely performed due to the lack of sensitivity in the detection methods available today [51, 52].
As mentioned, the cytopenias of ES in all 16 case reports involved anemia and thrombocytopenia. No occurrence of immune neutropenia was reported. In the literature, concurrent autoimmune neutropenia in ES was observed in only 10 of 68 adult patients (14.7%) and about 25% of children at the time of diagnosis, making it a relatively uncommon occurrence [13]. To add, in a systematic review on the clinical features of patients with ES, only 8.2% (18/219) cases reflected neutropenia [53]. Although neutrophils contain distinct antigenic profiles such as ANCA and NA1 that can be targeted in certain immune-mediated pathologies, their relatively short lifespan and being rapidly cleared from circulation most likely limits their exposure to immune-mediated destruction in the setting of ES [54].
A differential diagnosis is of vital importance for ES, as it is a diagnosis of exclusion. This requires ruling out similar diseases, including but not limited to thrombotic thrombocytopenia, cold agglutinin disease, infections, SLE, autoimmune lymphoproliferative syndrome, and malignancies, before suspecting ES [6]. Although all 16 patients included were diagnosed with ES, a thorough differential diagnosis was not reported in most cases. Future reports should, therefore, emphasize investigating differentials to reduce the chances of a false diagnosis in an already frequently misdiagnosed disorder [55].
Treatment regimens
As mentioned earlier, blood cell transfusions were used in seven of the 11 cases to correct the cytopenia [20, 24-28, 30]. While RBC transfusions are indicated for ES-associated AIHA when necessary, platelet transfusions for ES-associated thrombocytopenia are typically reserved for cases of life-threatening hemorrhages and used in conjunction with immunomodulatory drugs [51, 56-60]. In this review, platelet transfusions were administered in two cases; however, platelet count did not increase in response to the transfusion in any of these cases [25, 30]. In one of these cases, the patient passed away following the platelet transfusion [30]. With transfusions, there is always a risk of mid-transfusion fever, as seen in one of our cases, prompting the discontinuation of the transfusion and the administration of dexamethasone and IVIG [22]. Recent studies have reserved plasma exchange for life-threatening hemolysis in ITP or AIHA-associated ES [60-63].
The American Society of Hematology has not established specific guidelines for the treatment of ES, instead recommending a comprehensive, multi-faceted approach to management. Treatment decisions are influenced by several factors, including the severity of cytopenia, the presence of patient co-morbidities, the response to initial therapy, the risk of relapse, and the patient’s age [10, 64]. First-line treatments typically begin with corticosteroids, such as prednisone, administered at 1 - 2 mg/kg/day and tapered over time [10, 64]. IVIGs can serve as either a first-line or adjuvant therapy, particularly in patients presenting with thrombocytopenia [10, 64]. For second-line treatment, rituximab, is considered for refractory cases or patients experiencing persistent bleeding despite initial therapies [10, 64]. In more resistant cases, splenectomy, thrombopoietin receptor agonists, and hematopoietic stem cell transplantation may be indicated [10, 64].
The lack of evidence on the treatment of ES patients secondary to COVID-19 infection or immunization has made it difficult to establish standardized guidelines. Both the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend the use of corticosteroids, the first-line treatment for primary ES, for the treatment of severe or critical COVID-19, particularly in patients requiring supplemental oxygen or mechanical ventilation [65-68]. However, both organizations advise against the use of steroids in mild or non-severe cases, as the risks may outweigh the benefits in patients who do not require respiratory support [65-68]. This ambiguity in treatment creates significant complications and unforeseen events in patients with concurrent ES and COVID-19 symptoms. For instance, in one of the included reports, a patient with COVID-19, despite having a hemoglobin level of 6 g/dL, was given IVIG instead of corticosteroids [23]. After receiving a second dose of IVIG, the patient developed a popliteal deep venous thrombosis, which was treated with heparin. Consequently, IVIG was discontinued due to its suspected role in contributing to the thromboembolism.
The treatment regimen and the response to the treatment administered in the included case reports varied on a case-by-case basis. Therefore, further studies are needed to establish standardized treatment protocols, including expanding the FDA BEST monitoring protocol to also include AIHA post-vaccination (currently only ITP is listed among relevant parameters to ES) [69]. To establish appropriate laboratory monitoring intervals, a complete blood count (CBC) should be performed as soon as ES is suspected [70]. A Coombs test should be included in the initial workup to assess for hemolytic anemia [70]. Once ES is confirmed, weekly CBCs are advised to track blood counts until stabilization. Afterward, follow-up CBCs should be done every 2 - 4 weeks, with more frequent monitoring if signs of recurrence appear [70].
Limitations
Our study is not without limitations. First, due to variations in clinicians’ standards of care, there was subjectivity in the empirical data reported at presentation. This was evident in cases where hematological parameters were not available before admission, making baseline comparisons challenging. Additionally, hemoglobin levels and platelet counts were not always provided at patient discharge, hindering our ability to adequately assess treatment response. As a result, different parameters were selected for follow-up appointments.
Furthermore, the inconsistency in the diagnostic criteria for COVID-19 infection among the included case reports is a limitation. While some cases were confirmed via positive COVID-19 PCR or antigen tests, others were diagnosed based on clinical symptoms with a high suspicion of infection or chest X-ray impressions revealing a COVID-19 pattern of pneumonia.
Finally, one notable limitation of this study is the reliance on only 16 case reports, which restricts the ability to draw broad, generalizable conclusions. Studies with larger sample sizes are therefore necessary. This may be difficult as secondary ES can easily be overlooked and underreported in clinical practice. This underreporting highlights the critical need for prospective cohort studies or case-control investigations to better characterize ES secondary to COVID-19 infection/vaccination.
To expand the evidence base on ES secondary to post-COVID infection or vaccination, we call for international case reporting and collaboration. Sharing clinical cases from diverse populations worldwide will enhance our understanding of the prevalence, progression, and outcomes of the conditions, ultimately guiding more effective patient care and research.
| Conclusion | ▴Top |
This systematic review examined the clinical presentation, temporal relationship, diagnostics and treatment regimen of 16 cases of ES secondary to COVID-19 infection or vaccination. Patients with pre-existing autoimmune disorders require particular attention when investigating ES following COVID-19 vaccination or infection. Autoimmune conditions often involve a dysregulated immune system, which may increase the susceptibility to developing secondary autoimmune diseases like ES. While a causal relationship between ES and COVID-19 infection or vaccination cannot be determined at this stage due to data insufficiency, the evidence supports the temporality criterion, particularly between ES and COVID-19 vaccination. In terms of diagnostics, future cases of suspected ES post-COVID-19 infection or vaccination should also consider reporting peripheral blood smears, reticulocyte counts, and LDH levels. This will aid in formulating a more inclusive clinical picture of ES amid multiple differentials and comorbid autoimmune conditions. As for treatment, early initiation of corticosteroids and IVIG may be used as first-line therapies, given their frequent use and partial response rates observed in the reviewed cases, though standardized treatment protocols are needed to mitigate complications in the setting of COVID-19 pathogenesis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Study design: PR and AY; data collection: AY and MA; data extraction: AY, MA, and PR; manuscript writing: AY, MA, AH, and PR; manuscript revision: AY, MA, AH, and PR.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Fazeli P, Saeidnia M, Atefy N, Farzi A, Pooresmaeil N, Tamaddon G. Evans syndrome in the course of COVID-19 infection; essentials and approaches. IJBC. 2022;14(3):32-40.
- Abbas A, Helbawi F, Abdelsalam M. Treatment of People with Evans Syndrome in the Setting of COVID-19 Pandemic. Journal of Biomedical Research & Environmental Sciences. 2020;6:160-162.
doi - Chang R, Yen-Ting Chen T, Wang SI, Hung YM, Chen HY, Wei CJ. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine. 2023;56:101783.
doi pubmed - Sharma C, Bayry J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol. 2023;19(7):399-400.
doi pubmed - Yaamika H, Muralidas D, Elumalai K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J Taibah Univ Med Sci. 2023;18(6):1646-1661.
doi pubmed - Shaikh H, Mewawalla P. Evans Syndrome. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Michel M, Jager U. Chapter 46 - autoimmune hemolytic anemia. Editor(s): Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, Salama ME. Hematology (Seventh Edition). Elsevier. 2018; p. 648-662.e1.
doi - Aladjidi N, Fernandes H, Leblanc T, Vareliette A, Rieux-Laucat F, Bertrand Y, Chambost H, et al. Evans syndrome in children: long-term outcome in a prospective french national observational cohort. Front Pediatr. 2015;3:79.
doi pubmed - Schoonen WM, Kucera G, Coalson J, Li L, Rutstein M, Mowat F, Fryzek J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235-244.
doi pubmed - Fattizzo B, Michel M, Giannotta JA, Hansen DL, Arguello M, Sutto E, Bianchetti N, et al. Evans syndrome in adults: an observational multicenter study. Blood Adv. 2021;5(24):5468-5478.
doi pubmed - Hansen DL, Moller S, Andersen K, Gaist D, Frederiksen H. Evans syndrome in adults - incidence, prevalence, and survival in a nationwide cohort. Am J Hematol. 2019;94(10):1081-1090.
doi pubmed - Jaime-Perez JC, Aguilar-Calderon PE, Salazar-Cavazos L, Gomez-Almaguer D. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med. 2018;9:171-184.
doi pubmed - Michel M, Chanet V, Dechartres A, Morin AS, Piette JC, Cirasino L, Emilia G, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114(15):3167-3172.
doi pubmed - Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145-148.
doi pubmed - Mingot-Castellano ME, Butta N, Canaro M, Gomez Del Castillo Solano MDC, Sanchez-Gonzalez B, Jimenez-Barcenas R, Pascual-Izquierdo C, et al. COVID-19 vaccines and autoimmune hematologic disorders. Vaccines (Basel). 2022;10(6):961.
doi pubmed - Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a Systematic Review. SN Compr Clin Med. 2020;2(11):2048-2058.
doi pubmed - Taherifard E, Taherifard E, Movahed H, Mousavi MR. Hematologic autoimmune disorders in the course of COVID-19: a systematic review of reported cases. Hematology. 2021;26(1):225-239.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed - Oxford Centre for Evidence-Based Medicine. n.d.). Oxford Centre for Evidence-Based Medicine - Levels of Evidence. 2009. Retrieved January 28, 2022, from https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-forevidencebased-medicine-levels-of-evidence-march-2009.
- Zarza J, Von Horoch J, Aguayo N, Baez E. Evans syndrome associated with antiphospholipid antibodies in a patient with SARS-COV-2 infection. Hematol Transfus Cell Ther. 2020;42(4):309-312.
doi pubmed - Turgutkaya A, Bolaman AZ, Yavasoglu I. COVID-19-associated Evans syndrome: A case report and review of the literature. Transfus Apher Sci. 2022;61(3):103339.
doi pubmed - Shah P, Kela K, Sharma AM, Jain KN, Sharma N. COVID-19-induced Evans syndrome: unusual complication of the new usual. Chest. 2022;162(4):A627.
doi - Li M, Nguyen CB, Yeung Z, Sanchez K, Rosen D, Bushan S. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190(2):e59-e61.
doi pubmed - Demir NA, Basturk A, Ural O, Sumer S, Erdogdu B, Kiratli HE, Celik JB, et al. A case of Evans syndrome secondary to COVID-19. Blood Transfus. 2021;19(1):85-88.
doi pubmed - Santosa D, Sofro MAU, Farida, Nindita N, Pangarsa EA, Setiawan B, Rizky D, et al. A full-term pregnant woman with secondary Evans syndrome caused by severe coronavirus disease 2019: a case report. J Med Case Rep. 2021;15(1):606.
doi pubmed - Mohammadien HA, Abudab LH, Ahmad AM. Evan syndrome as initial presentation of COVID-19 infection. The Egyptian Journal of Bronchology. 2022;16(1):22.
doi - Wahlster L, Weichert-Leahey N, Trissal M, Grace RF, Sankaran VG. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr Blood Cancer. 2020;67(9):e28382.
doi pubmed - Zama D, Pancaldi L, Baccelli F, Guida F, Gottardi F, Dentale N, Esposito F, et al. Autoimmune hemolytic anemia in children with COVID-19. Pediatr Blood Cancer. 2022;69(2):e29330.
doi pubmed - Ghariani I, Braham NJ, Bekir L. [Evans syndrome as initial presentation of COVID-19 infection: A case report and review of the literature]. Ann Biol Clin (Paris). 2023;81(1):91-95.
doi pubmed - Georgy JT, Jayakaran JAJ, Jacob AS, Gunasekaran K, Korula PJ, Devasia AJ, Iyadurai R. Evans syndrome and immune thrombocytopenia in two patients with COVID-19. J Med Virol. 2021;93(5):2642-2644.
doi pubmed - Hidaka D, Ogasawara R, Sugimura S, Fujii F, Kojima K, Nagai J, Ebata K, et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int J Hematol. 2022;115(3):424-427.
doi pubmed - De Felice M, Farina G, Bianco R, Monaco G, Iaccarino S. Evans Syndrome Presenting as an Atypical Complication of SARS-CoV-2 Vaccination. Cureus. 2022;14(7):e26602.
doi pubmed - Gambichler T, Nordmann P, Scheel C, Susok L. Evans' syndrome following vaccination with ChAdOx1 nCoV-19 in a patient with new-onset localized scleroderma. Dermatol Reports. 2022;14(4):9470.
doi pubmed - Ng TYM, Teo WZY, Ng TYM, Teng GG. New-onset Evans syndrome in a patient with SLE post SARS-CoV2 mRNA vaccination. Ann Hematol. 2023;102(1):235-236.
doi pubmed - Cvetkovic M, Pantic N, Virijevic M, Pravdic Z, Sabljic N, Mitrovic M, Suvajdzic-Vukovic N. Relapse of Evans syndrome following BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine: case report and literature review. J Infect Dev Ctries. 2023;17(6):800-804.
doi pubmed - Centers of Disease Control. Symptoms of COVID-19. 2022. Retrieved from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- Jaime-Perez JC, Guerra-Leal LN, Lopez-Razo ON, Mendez-Ramirez N, Gomez-Almaguer D. Experience with Evans syndrome in an academic referral center. Rev Bras Hematol Hemoter. 2015;37(4):230-235.
doi pubmed - Deleze M, Oria CV, Alarcon-Segovia D. Occurrence of both hemolytic anemia and thrombocytopenic purpura (Evans' syndrome) in systemic lupus erythematosus. Relationship to antiphospholipid antibodies. J Rheumatol. 1988;15(4):611-615.
pubmed - Adams RM. Evans syndrome treatment & management. Medscape. 2024. https://emedicine.medscape.com/article/955266-treatment#d12.
- Mahroum N, Lavine N, Ohayon A, Seida R, Alwani A, Alrais M, Zoubi M, et al. COVID-19 vaccination and the rate of immune and autoimmune adverse events following immunization: insights from a narrative literature review. Front Immunol. 2022;13:872683.
doi pubmed - Al-Beltagi M, Saeed NK, Bediwy AS. COVID-19 disease and autoimmune disorders: A mutual pathway. World J Methodol. 2022;12(4):200-223.
doi pubmed - Kayal L, Jayachandran S, Singh K. Idiopathic thrombocytopenic purpura. Contemp Clin Dent. 2014;5(3):410-414.
doi pubmed - Hill A, Hill QA. Autoimmune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2018;2018(1):382-389.
doi pubmed - Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577-582.
doi pubmed - Angileri F, Legare S, Marino Gammazza A, Conway de Macario E, Macario AJL, Cappello F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol. 2020;190(2):e92-e93.
doi pubmed - Fattizzo B. Evans syndrome and infections: a dangerous cocktail to manage with caution. Blood Transfus. 2021;19(1):5-8.
doi pubmed - Shlamovitz GZ, Johar S. A case of Evans' syndrome following influenza vaccine. J Emerg Med. 2013;44(2):e149-151.
doi pubmed - Martinez E, Domingo P. Evans's syndrome triggered by recombinant hepatitis B vaccine. Clin Infect Dis. 1992;15(6):1051.
doi pubmed - Marschalek R. The long-COVID syndrome: neoantigens as driving force for the onset of autoimmune diseases. Journal of Cellular Immunology. 2025;7:26-31.
doi - Medscape. Evans Syndrome Workup. 2020. Retrieved from: https://emedicine.medscape.com/article/955266-workup#c7.
- Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, Ghanima W, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817.
doi pubmed - Porcelijn L, Schmidt DE, Oldert G, Hofstede-van Egmond S, Kapur R, Zwaginga JJ, de Haas M. Evolution and utility of antiplatelet autoantibody testing in patients with immune thrombocytopenia. Transfus Med Rev. 2020;34(4):258-269.
doi pubmed - Sugam Gouli, Ojbindra KC, Mariam Mostafa, Asis Shrestha, Sujan Niraula, Aliza Dulal. Evans syndrome: a systematic review. Blood. 2024;144(Supplement 1):7720.
doi - Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127(18):2173-2181.
doi pubmed - Al Hazmi A, Winters ME. Evans syndrome. Clin Pract Cases Emerg Med. 2019;3(2):128-131.
doi pubmed - Jager U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, Jilma B, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648.
doi pubmed - Hill QA, Stamps R, Massey E, Grainger JD, Provan D, Hill A, British Society for H. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017;176(3):395-411.
doi pubmed - Goel R, Chopra S, Tobian AAR, Ness PM, Frank SM, Cushing M, Vasovic L, et al. Platelet transfusion practices in immune thrombocytopenia related hospitalizations. Transfusion. 2019;59(1):169-176.
doi pubmed - Spahr JE, Rodgers GM. Treatment of immune-mediated thrombocytopenia purpura with concurrent intravenous immunoglobulin and platelet transfusion: a retrospective review of 40 patients. Am J Hematol. 2008;83(2):122-125.
doi pubmed - Audia S, Grienay N, Mounier M, Michel M, Bonnotte B. Evans' syndrome: from diagnosis to treatment. J Clin Med. 2020;9(12):3851.
doi pubmed - Ruivard M, Tournilhac O, Montel S, Fouilhoux AC, Quainon F, Lenat A, Travade P, et al. Plasma exchanges do not increase red blood cell transfusion efficiency in severe autoimmune hemolytic anemia: a retrospective case-control study. J Clin Apher. 2006;21(3):202-206.
doi pubmed - von Baeyer H. Plasmapheresis in immune hematology: review of clinical outcome data with respect to evidence-based medicine and clinical experience. Ther Apher Dial. 2003;7(1):127-140.
doi pubmed - Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, Pham HP, et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American society for apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171-354.
doi pubmed - Norton A, Roberts I. Management of Evans syndrome. Br J Haematol. 2006;132(2):125-137.
doi pubmed - World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. Retrieved from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- Centers for Disease Control. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 2020. Retrieved from: https://stacks.cdc.gov/view/cdc/89980.
- Centers for Disease Control and Prevention. Updated information on availability and use of treatments for outpatients with mild to moderate COVID-19 who are at increased risk for severe outcomes of COVID-19. 2022. https://archive.cdc.gov/www_cdc_gov/han/2022/han00463.html.
- World Health Organization. Corticosteroids for COVID-19: Living guidance. 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1.
- BEST Initiative. COVID-19 vaccine safety active monitoring protocol. Biologics Effectiveness and Safety (BEST) Initiative. 2020. https://bestinitiative.org/wp-content/uploads/2020/12/C19-Vaccine-Safety-Protocol-2020.pdf.
- Adams RM. Evans syndrome treatment & management. Medscape. 2024. https://emedicine.medscape.com/article/955266-treatment#d12.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.