| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Original Article
Volume 000, Number 000, May 2025, pages 000-000
Efficacy of Short-Course High-Dose Oral Prednisolone in Rapid Platelet Recovery for Pediatric Acute Immune Thrombocytopenic Purpura: A Prospective Cohort Study
Akshat Jhingana , Neha Goela
, Amitabh Singha, c
, Rani Geraa, c
, Nidhi Chopraa
, Sumit Mehndirattaa
, Monica Sharmab
aDepartment of Pediatrics, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi 110029, India
bDepartment of Hematology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi 110029, India
cCorresponding Author: Amitabh Singh and Rani Gera, Department of Pediatrics, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi 110029, Indiaand
Manuscript submitted February 27, 2025, accepted April 30, 2025, published online May 13, 2025
Short title: High-Dose Oral PDN in Pediatric Acute ITP
doi: https://doi.org/10.14740/jh2052
| Abstract | ▴Top |
Background: Standard management of acute immune thrombocytopenic purpura (ITP) remains debated, with some advocating observation for mild cases, while others recommend pharmacological intervention to accelerate platelet recovery in children with severe thrombocytopenia (TCP) (platelet count < 20,000/mm3) or significant mucosal bleeds. Corticosteroids, particularly prednisolone (PDN), are a widely used first-line treatment due to their rapid immunosuppressive effect. This prospective cohort study evaluated the effectiveness of short-course high-dose PDN (4 mg/kg/day for 7 days) in treating children aged 1 - 12 years with newly diagnosed acute ITP. The study aimed to assess the clinical and hematological profile of these children and the mean time to platelet recovery.
Methods: A total of 61 patients were enrolled, and their response to treatment was monitored at various intervals, including 48 h, 72 h, day 7, 1 month, and 3 months.
Results: Results revealed a rapid platelet recovery in patients receiving high-dose PDN, with 83.6% of patients achieving platelet counts greater than 50,000/mm3 by day 7. Additionally, 91.8% maintained platelet recovery at 1 month. The study also found that the age group 6 - 12 years had a higher risk of persistent ITP (24.5%), highlighting the importance of close monitoring in this demographic. While the treatment was generally well tolerated, some mild side effects like hypertension and glycosuria were observed.
Conclusion: The study suggests that short-course high-dose PDN can be an effective first-line therapy for acute ITP. It promotes faster platelet recovery and reduces hospitalization time with minimal adverse effects.
Keywords: Immune thrombocytopenic purpura; Thrombocytopenia; Corticosteroids; Prednisolone
| Introduction | ▴Top |
Immune thrombocytopenic purpura (ITP) is a self-limiting autoimmune disorder characterized by thrombocytopenia (TCP), increased platelet destruction, and impaired platelet production, often triggered by a preceding viral infection [1]. While ITP in children typically resolves spontaneously, some cases present with significant mucosal bleeding, necessitating prompt intervention to reduce the risk of life-threatening hemorrhages, such as intracranial hemorrhage (ICH) [2]. The standard management of acute ITP remains debated, with some advocating observation for mild cases, while others recommend pharmacological intervention to accelerate platelet recovery in children with severe TCP (platelet count < 20,000/mm3) or significant mucosal bleeds [3]. Corticosteroids, particularly prednisolone (PDN), are a widely used first-line treatment due to their rapid immunosuppressive effect. Standard PDN therapy (2 mg/kg/day for 14 days) effectively increases platelet counts but may take a week or more to achieve a hemostatic level [4]. Recent studies suggest that high-dose PDN (4 mg/kg/day) may induce a more rapid platelet rise, potentially reducing the risk of hemorrhagic complications within the first crucial days of treatment. However, data on the mean time to platelet recovery with short-course, high-dose PDN remain limited [5]. The American Society of Hematology (ASH) guideline panel also recommends against corticosteroid courses exceeding 7 days in children with newly diagnosed ITP and non-life-threatening bleeding. This strong recommendation is based on very low certainty evidence of benefits and high certainty evidence of harm, considering the low risk of bleeding, high spontaneous remission rates in children, and lack of evidence supporting long-term corticosteroid use [6]. This prospective study aimed to evaluate the clinical-hematological profile and mean time to platelet recovery in children aged 1 - 12 years with newly diagnosed acute ITP receiving a short course of high-dose oral PDN (4 mg/kg/day for 7 days) [6].
| Materials and Methods | ▴Top |
Study design and setting
This prospective cohort study was conducted over 18 months (July 2020 to December 2021) at a tertiary care hospital in New Delhi, India.
Study population
A total of 61 newly diagnosed pediatric patients (1 - 12 years) with acute ITP were enrolled. Participants were recruited from both inpatient and outpatient departments after parents/guardians provided informed consent.
Inclusion and exclusion criteria
Inclusion criteria were: children aged 1 - 12 years with newly diagnosed acute primary ITP; WHO grade 3 or 4 mucosal bleeding requiring intervention.
Exclusion criteria were: secondary causes of ITP; grade 1 or 2 bleeding not requiring treatment; prior ITP treatment from other healthcare facilities; chronic or persistent ITP at presentation; parental refusal to participate.
Definitions are shown in Supplementary Material 1 (jh.elmerpub.com) [6].
Treatment protocol
Our hospital’s initial standard of care (SOC) for pediatric acute ITP was to give oral PDN at 2 mg/kg/day for 2 weeks with a slow taper in patients with grade 3-4 mucosal bleed without life-threatening hemorrhage and to give intravenous immunoglobulin (IVIG) (0.8 g/kg as a single dose), followed by oral PDN (2 mg/kg/day for 2 weeks followed by a slow taper) in patients with severe bleeding (e.g., life-threatening ICH or gastrointestinal hemorrhage). For this study, the patients have been divided into two groups as follows: 1) High-dose PDN group: Patients with grade 3-4 mucosal bleeding without life-threatening hemorrhage received oral PDN (4 mg/kg/day for 7 days). 2) IVIG group: Patients with severe bleeding (e.g., life-threatening ICH or gastrointestinal hemorrhage) received IVIG (0.8 g/kg as a single dose), followed by oral PDN (2 mg/kg/day for 2 weeks followed by a slow taper). 3) Platelet transfusions were reserved for patients with severe bleeding (gastrointestinal or intracerebral hemorrhage).
Outcome measures
Primary outcome was mean time to platelet recovery (> 30,000/mm3). Secondary outcomes were platelet count trends at 0 h, 48 h, 72 h, day 7, 1 month, and 3 months; rate of bleeding cessation; adverse effects of steroid therapy.
Statistical analysis
Where appropriate, categorical variables were analyzed using the Chi-square test or Fisher’s exact test. Quantitative data were analyzed using independent t-tests and summarized as mean ± standard deviation (SD) or median (interquartile range (IQR)). Statistical significance was set at P < 0.05. Data analysis was conducted using SPSS version 25.0 (IBM, Chicago, USA).
Ethical considerations
Ethical approval was obtained from the Institutional Review Board of Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi (approval no: IEC/VMMC/SJH/Thesis/2020-11/CC-210). Written informed consent was obtained from parents/guardians, and the study adhered to the ethical principles outlined in the Declaration of Helsinki.
| Results and Discussion | ▴Top |
This prospective cohort study evaluated the clinical-hematological profile and platelet recovery time in children aged 1 - 12 years with newly diagnosed primary acute ITP receiving short-course high-dose oral PDN. A total of 61 patients were enrolled, with a nearly equal distribution of males (52.46%) and females (47.54%). The majority of cases (57.38%) belonged to the 1 - 5 years age group, consistent with the known peak incidence of pediatric ITP as shown by Neunert et al [6]. A preceding viral infection was reported in 49.18% of cases, a finding in line with previous literature suggesting a post-viral autoimmune mechanism in pediatric ITP by Rodeghiero et al [7]. Baseline demographic and clinical profile of patients is shown in Table 1.
 Click to view | Table 1. Basic Demographic and Clinical Profile of Pediatric Acute ITP Patients |
Platelet recovery and response to treatment
Patients receiving high-dose PDN (4 mg/kg/day for 7 days) demonstrated a rapid rise in platelet counts. At 48 h, 37.7% of patients achieved a platelet count > 30,000/mm3; by 72 h, 62.3% reached this threshold. On day 7, 83.6% of cases had platelet counts of > 50,000/mm3, while at 1 month, 91.8% of cases had sustained platelet recovery. These findings align with earlier studies suggesting that high-dose corticosteroids induce a faster platelet response compared to standard-dose regimens done by Praituan et al [8]. By 3 months, complete remission was observed in 75.5% of cases.
Descriptive statistics of platelet counts at day 0, day 2, day 3, day 8, 1 month, and 3 months are shown in Figure 1a, and a comparison of platelet counts (cells/mm3) between high-dose PDN and IVIG is shown in Figure 1b.
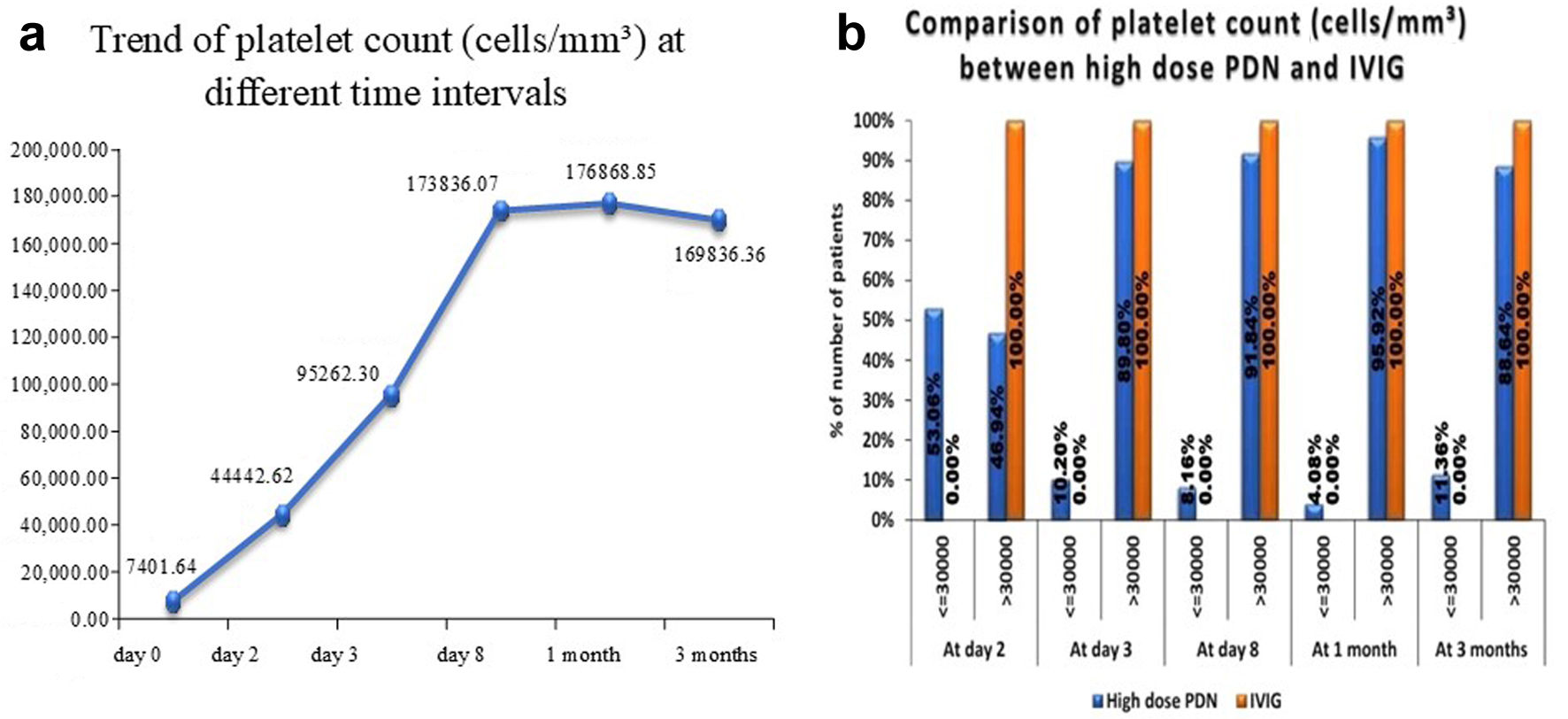 Click for large image | Figure 1. (a) Descriptive statistics of platelet count at day 0, day 2, day 3, day 8, 1 month, and 3 months of study subjects. (b) Comparison of platelet counts (cells/mm3) between high-dose PDN and IVIG. IVIG: intravenous immunoglobulin; PDN: prednisolone. |
A comparison of the time taken to attain the absence of bleeding and the mean time to recover (in hours) between high-dose PDN and IVIG is shown in Table 2. IVIG patients took less time to recover, and IVIG can be considered superior to high-dose PDN as literature has suggested that time to initial response in case of IVIG is 1 - 3 days while that for PDN at 1 - 4 mg/kg/day for 1 - 4 weeks is 4 - 14 days [7]; however, comparative analysis of IVIG and PDN revealed minimal benefits with IVIG in terms of durable response, remission, bleeding event prevention, or mortality between the two treatments. Also, IVIG is associated with higher costs, the need for inpatient admission and intravenous access and limited availability compared to corticosteroids. Consequently, the 2019 American Society of Hematology (ASH) ITP guidelines recommend corticosteroids over IVIG for children with newly diagnosed ITP and non-life-threatening bleeding [6].
 Click to view | Table 2. Comparison of Time Taken to Attain Absence of Bleeding and Mean Time to Recover Between High-Dose PDN and IVIG |
Persistent ITP and risk factors
Persistent ITP, defined as TCP lasting beyond 3 months, was observed in 15 of 61 cases (24.5%), out of which 14 patients received high-dose PDN and one patient received IVIG (P = 0.262), which is comparable to previous studies reporting persistence rates of 20-30% by Rodeghiero et al [8] and Neunert et al [6]. Since P > 0.05, the result is not statistically significant, there is no substantial evidence that the choice of treatment (high-dose PDN vs. IVIG) significantly affects the likelihood of developing persistent ITP. The proportion of persistent ITP was significantly lower in the 1 - 5 years age group (13.33%) compared to the 6 - 12 years group (44%) (P = 0.016), reinforcing prior evidence that older age is a predictor of chronicity [9]. Additionally, the mean age of patients with persistent ITP was significantly higher (6.87 ± 2.1 years, P = 0.014). A history of past viral infection was present in only 11.1% of persistent ITP cases, indicating a potential protective role of antecedent infections in triggering a transient immune response rather than prolonged autoimmunity. However, multivariate logistic regression did not identify any independent risk factors (age, platelet count at day 0, 2, 3 or previous history of viral illness) for persistent ITP, suggesting that chronicity may result from a multifactorial interplay of immune dysregulation and genetic predisposition [9].
Table 3 shows the association of various significant risk factors with persistent ITP. However, on performing multivariate logistic regression, none of the variables were independent risk factors for persistent ITP.
 Click to view | Table 3. Multivariate Logistic Regression to Find Out Significant Risk Factors of Persistent ITP |
Complications following treatment
Adverse effects of high-dose PDN were minimal. Hypertension was observed in three of 61 patients (4.92%), while glycosuria was detected in seven of 61 patients (11.48%), which is consistent with known corticosteroid side effects reported by Bennett et al [10]. Importantly, no life-threatening complications such as sepsis or severe infections were observed during follow-up.
Comparison with existing literature
The efficacy of high-dose PDN in achieving rapid platelet recovery aligns with previous findings by Kuhne et al (2011) [11], who reported a median time to response of 2 - 3 days with high-dose corticosteroids. Furthermore, our observed persistence rate of 24.5% is consistent with the range reported in other cohort studies (20-30%) by Neunert et al [6]. The association of older age with persistent ITP corroborates findings by Heitink-Polle et al [9], emphasizing the need for close monitoring of older children with newly diagnosed ITP.
Clinical implications
The findings highlight that a short-course high-dose PDN regimen can be an effective first-line therapy for acute ITP, leading to a rapid platelet response and reducing hospitalization time. However, given that 24.5% of cases progressed to persistent ITP, clinicians should identify high-risk groups (e.g., older children) for closer follow-up. Moreover, while corticosteroid side effects were generally mild, clinicians should monitor for metabolic disturbances in prolonged or repeated steroid therapy.
Conclusion
Short-course high-dose PDN (4 mg/kg/day for 7 days) effectively promotes rapid platelet recovery in pediatric acute ITP, with a favorable safety profile. The study reinforces that age ≥ 6 years is associated with a higher risk of persistent ITP, necessitating tailored follow-up strategies. Further multicenter studies with larger cohorts are needed to validate these findings and refine treatment protocols.
| Supplementary Material | ▴Top |
Suppl 1. Definitions of terms in 2019 ASH guidelines on ITP.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent has been taken from the parents at the time of enrollment of the patient in the study.
Author Contributions
Each author contributed equally to the preparation of the manuscript. AS and RG conceptualized and designed the manuscript. AJ, NG, and AS conducted the literature search. AJ and AS acquired data. NG, RG, MS, and AS prepared and edited the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ICH: intracranial hemorrhage; IPD: inpatient department; ITP: immune thrombocytopenic purpura; OPD: outpatient department; PDN: prednisolone; TCP: thrombocytopenia
| References | ▴Top |
- Schulman I. Diagnosis and treatment: management of idiopathic thrombocytopenic purpura. Pediatrics. 1964;33:979-980.
pubmed - Wilson DB. Acquired platelet disorders. Nathan and Oski-Textbook of haematology of infancy and childhood. 6th ed. p. 1600-1610.
- Arnold DM. Platelet count or bleeding as the outcome in ITP trials? Am J Hematol. 2012;87(10):945-946.
doi pubmed - Buchanan GR, Holtkamp CA. Prednisone therapy for children with newly diagnosed idiopathic thrombocytopenic purpura. A randomized clinical trial. Am J Pediatr Hematol Oncol. 1984;6(4):355-361.
doi pubmed - Lilleyman JS. Platelet disorders. Pediatric Hematology. 2nd ed. p. 442-447.
- Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866.
doi pubmed - Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
doi pubmed - Praituan W, Rojnuckarin P. Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 2009;7(6):1036-1038.
doi pubmed - Heitink-Polle KM, Nijsten J, Boonacker CW, de Haas M, Bruin MC. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood. 2014;124(22):3295-3307.
doi pubmed - Bennett CM, Neunert C, Grace RF, et al. Chronic immune thrombocytopenia in children: a longitudinal cohort study. Journal of Pediatrics. 2013;163(6):1530-1536.
- Kuhne T, Berchtold W, Michaels LA, Wu R, Donato H, Espina B, Tamary H, et al. Newly diagnosed immune thrombocytopenia in children and adults: a comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96(12):1831-1837.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.