| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 000, Number 000, April 2025, pages 000-000
Hairy Cell Leukemia With Splenic Rupture: Hematological Changes and Disappearance of Hairy Morphology After Splenectomy
Stanley Kima, d , Anna Mikamib, William Stullc
aDivision of Hematology and Medical Oncology, Department of Medicine, Kern Medical, Bakersfield, CA 93306, USA
bCollege of Osteopathic Medicine - Northwest, Western University of Health Science, Lebanon, OR 97355, USA
cDepartment of Pathology, Kern Medical, Bakersfield, CA 93306, USA
dCorresponding Author: Stanley Kim, Division of Hematology and Medical Oncology, Department of Medicine, Kern Medical, Bakersfield, CA 93306, USA
Manuscript submitted February 13, 2025, accepted April 4, 2025, published online April 16, 2025
Short title: Hairy Cell Leukemia With Splenic Rupture
doi: https://doi.org/10.14740/jh2045
| Abstract | ▴Top |
We present the case of a 64-year-old man who suffered a splenic rupture following a fall. A peripheral blood smear showed mononuclear cells with “hairy” cytoplasmic projections. A computed tomography (CT) angiogram revealed splenomegaly with a hematoma and active contrast extravasation. He underwent coil embolization of the splenic artery but subsequently developed worsening abdominal pain and ileus, requiring a splenectomy. Pathological examination of the spleen showed extensive infiltration by hairy cell leukemia (HCL). Bone marrow biopsy revealed hypercellular marrow predominantly infiltrated by HCL cells positive for CD20, CD103, and the BRAF V600E mutation. After the splenectomy, pancytopenia gradually improved. The symptoms of the patient, such as fatigue, weight loss, and night sweats, were also resolved. Circulating HCL cells were significantly reduced, and the “hairy” morphology became smoother. To our knowledge, this morphologic change of the hairy cells after splenectomy has not been reported in the past. Its mechanism is not known. We postulate that splenectomy may induce molecular or immunological changes that alter HCL cell behavior, which warrants further research.
Keywords: Hairy cell leukemia; Splenectomy; Hairy cell morphology
| Introduction | ▴Top |
Hairy cell leukemia (HCL) is a rare chronic B-cell leukemia characterized by pancytopenia, monocytopenia, splenomegaly, and the presence of “hairy” cytoplasmic projections on leukemic cells. Diagnosis relies on peripheral blood smear findings, bone marrow examination, flow cytometry, and immunophenotypic features such as the expression of CD11c, CD103, CD123, CD25, and the identification of the BRAF V600E somatic mutation [1].
Patients with HCL commonly present with fatigue, recurrent infections, and splenomegaly. Asymptomatic cases may not require immediate treatment. For symptomatic patients, the first-line treatment involves purine nucleoside analogs (cladribine or pentostatin), often combined with rituximab. For recurrent or refractory disease, therapeutic options include BRAF inhibitors, immunoconjugates, Bruton tyrosine kinase inhibitors, or interferon-alpha (IFN-α) [1].
Historically, splenectomy was the primary treatment for HCL before the introduction of more effective therapies such as IFN-α in the mid-1980s. While splenectomy improves blood cell counts by removing the spleen’s sequestration function, it does not improve bone marrow infiltration and fibrosis, and positive influences of splenectomy with regard to long-term results are reported to be uncertain [2]. Splenectomy is not without morbidity; complications occur in 17.0% and the operative mortality rate is 1.6% [3].
Hairy projections on the cell surface are characteristic of HCL cells but are also observed in other HCL-like diseases, including the HCL variant (splenic B-cell lymphoma/leukemia with prominent nucleoli), splenic marginal zone lymphoma, and splenic diffuse red pulp lymphoma [4].
We present the case of an elderly man with HCL who underwent splenectomy for a traumatic splenic rupture. We also explore post-splenectomy hematological changes, including alterations in hairy cell morphology, and discuss the potential therapeutic benefits of splenectomy as a treatment option for HCL.
| Case Report | ▴Top |
A 64-year-old Caucasian man with a medical history of hypertension presented to our hospital with severe left upper abdominal pain after a fall due to syncope. The patient said he had experienced fatigue, weight loss, and night sweats for the past 6 months. On arrival, his physical examination showed a blood pressure (BP) of 153/83 mm Hg, a pulse of 102/min, a respiratory rate of 22/min, no fever, and left upper quadrant tenderness. The spleen was not palpable due to guarding. White blood cell (WBC) count was 10.8 × 103/µL, hemoglobin (Hb) level was 9.0 g/dL, and platelet count was 73 × 103/µL (Table 1). Blood urea nitrogen (BUN) level was 28 mg/dL, creatinine level was 1.11 mg/dL, albumin measured 2.8 g/dL, alkaline phosphatase was 189 IU/L, alanine aminotransferase (ALT) was 102 IU/L, aspartate aminotransferase (AST) was 34 IU/L, and total bilirubin was 1.3 mg/dL. The peripheral blood smear showed large mononuclear cells with hairy projections comprising about 20% of WBCs (Fig. 1a). Computed tomography (CT) scan of the abdomen (Fig. 2) showed an enlarged spleen (19 cm in length) and a capsular hematoma (3.3 cm in thickness). Because the CT angiogram showed contrast substance extravasation within the inferior aspect of the spleen, he underwent urgent coil embolization of the spleen and gastroepiploic arteries. After the procedure, he developed abdominal distension, nausea, vomiting, hiccupping, and an ileus requiring prolonged nasogastric tube placement. For increasing abdominal pain, persistent ileus, and CT findings of necrotic spleen with gas formation, a decision was made to proceed with splenectomy on postembolization day 13. The patient received vaccinations for Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae prior to surgery. During surgery, 6 L of hemorrhagic ascites was evacuated, and the patient was given four units of packed red blood cell transfusion. The removed spleen was ruptured, necrotic, and enlarged, measuring 21 × 18 × 8 cm and weighing 1,503 g (Fig. 3). The spleen was infiltrated by HCL cells positive for CD20 and CD103. Immunohistochemical (IHC) stain for mutant BRAF V600E protein (reagent: monoclonal antibody IHC600) was also positive (Fig. 4a, b). The bone marrow biopsy showed hypercellular marrow (90%), mostly with positive CD20 and CD103 mononuclear cells with a fried egg appearance (Fig. 5a-c) and markedly increased reticulin fibrosis. The flow cytometry performed on the bone marrow aspirates included CD11c, CD25, and CD103, all of which were positive in the B cells. The IHC study for CD123 was not performed.
 Click to view | Table 1. Changes in Blood Cells and Hairy Cells After Splenectomy |
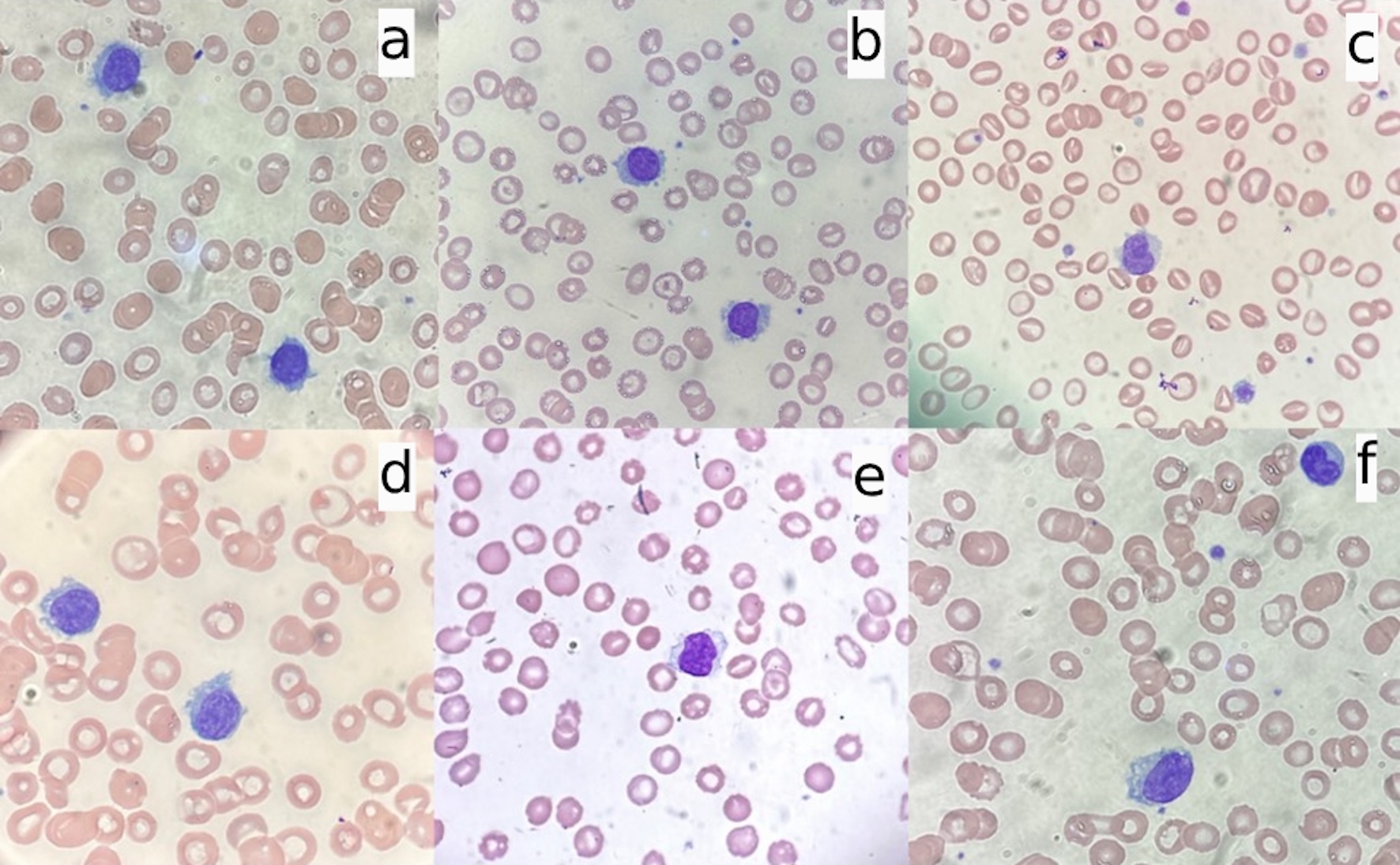 Click for large image | Figure 1. Morphology changes of hairy cells before and after splenectomy (× 100). (a) Before splenectomy; (b) post-splenectomy day 7; (c) post-splenectomy day 13; (d) post-splenectomy day 26; (e) post-splenectomy day 54; (f) post-splenectomy day 84. |
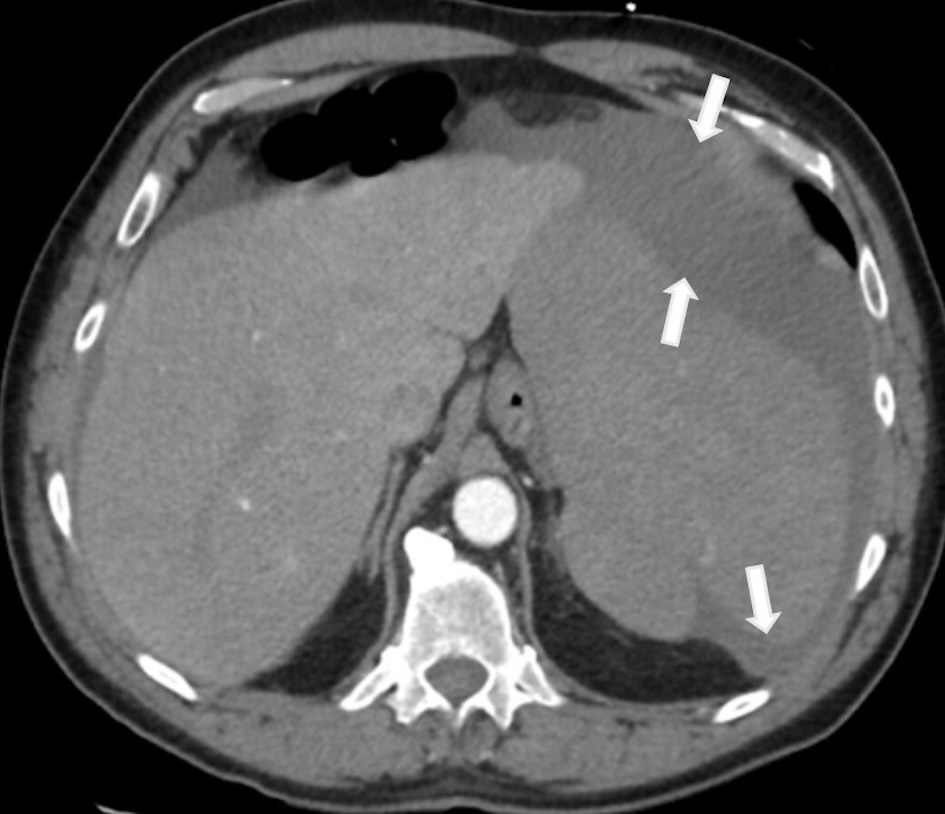 Click for large image | Figure 2. CT scan showing an enlarged spleen with capsular hematomas (arrows). CT: computed tomography. |
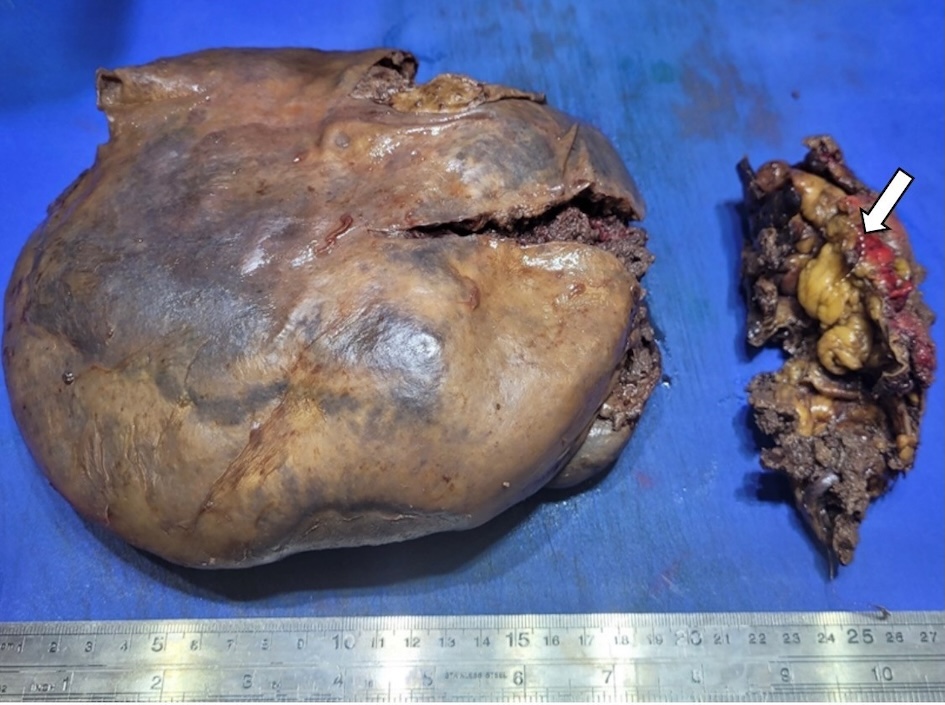 Click for large image | Figure 3. Removed spleen showing enlarged, ruptured, and necrotic spleen with the embolization coil (arrow). |
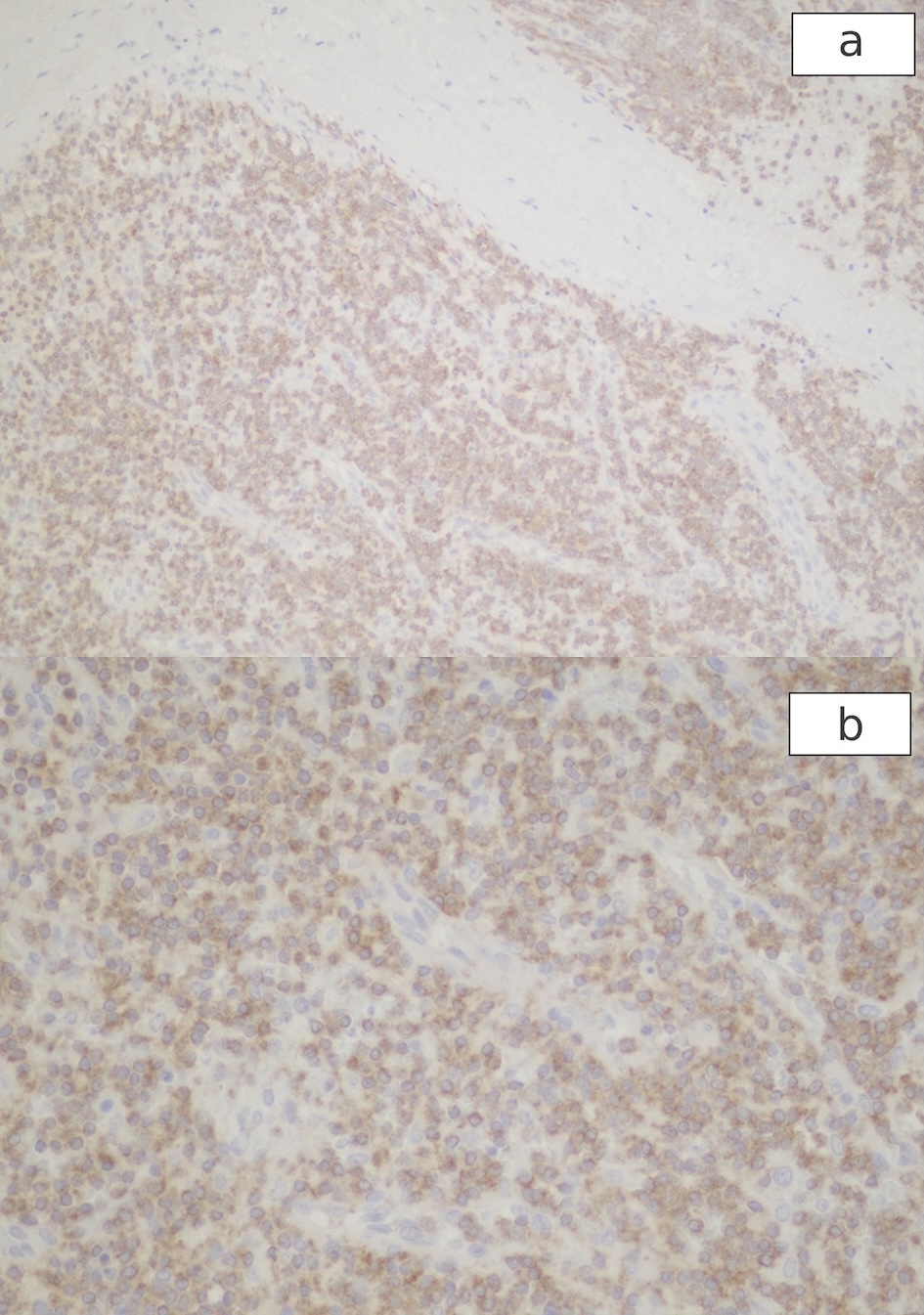 Click for large image | Figure 4. Positive immunohistochemical stain for mutant BRAF V600E protein in the cytoplasm of HCL cells of the spleen. (a): × 10; (b): × 20. HCL: hairy cell leukemia. |
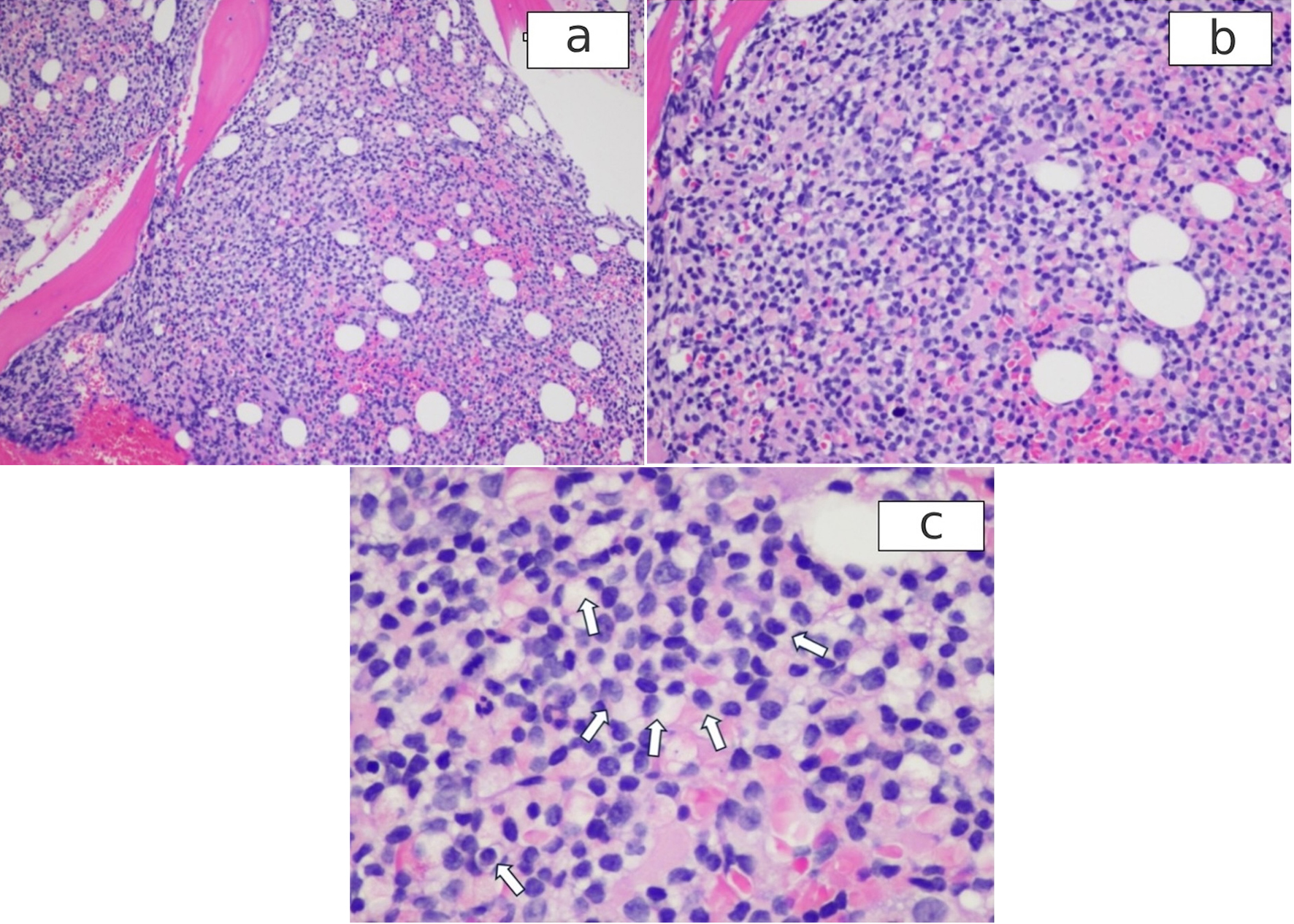 Click for large image | Figure 5. H&E stained bone marrow shows the infiltrates by HCL. (a): × 10; (b): × 20; (c): magnifying view of the bone marrow showing fried egg appearance of HCL cells (arrows). HCL: hairy cell leukemia; H&E: hematoxylin and eosin. |
Outcome and follow-up
The postsurgical course was complicated by pneumonia and gastrointestinal bleeding, which were managed successfully with antibiotics, pantoprazole, and blood transfusion. He also developed drug skin eruption, apparently from piperacillin-tazobactam, which was switched to cefepime. The skin rashes faded significantly. The patient was discharged on post-surgery day 13. Complete blood count was obtained daily during hospitalization and biweekly after discharge for 4 months, and peripheral blood smears were examined daily during hospitalization and biweekly after discharge to observe the changes in number and morphology of the circulating hairy cells (Table 1, Figs. 1, 6).
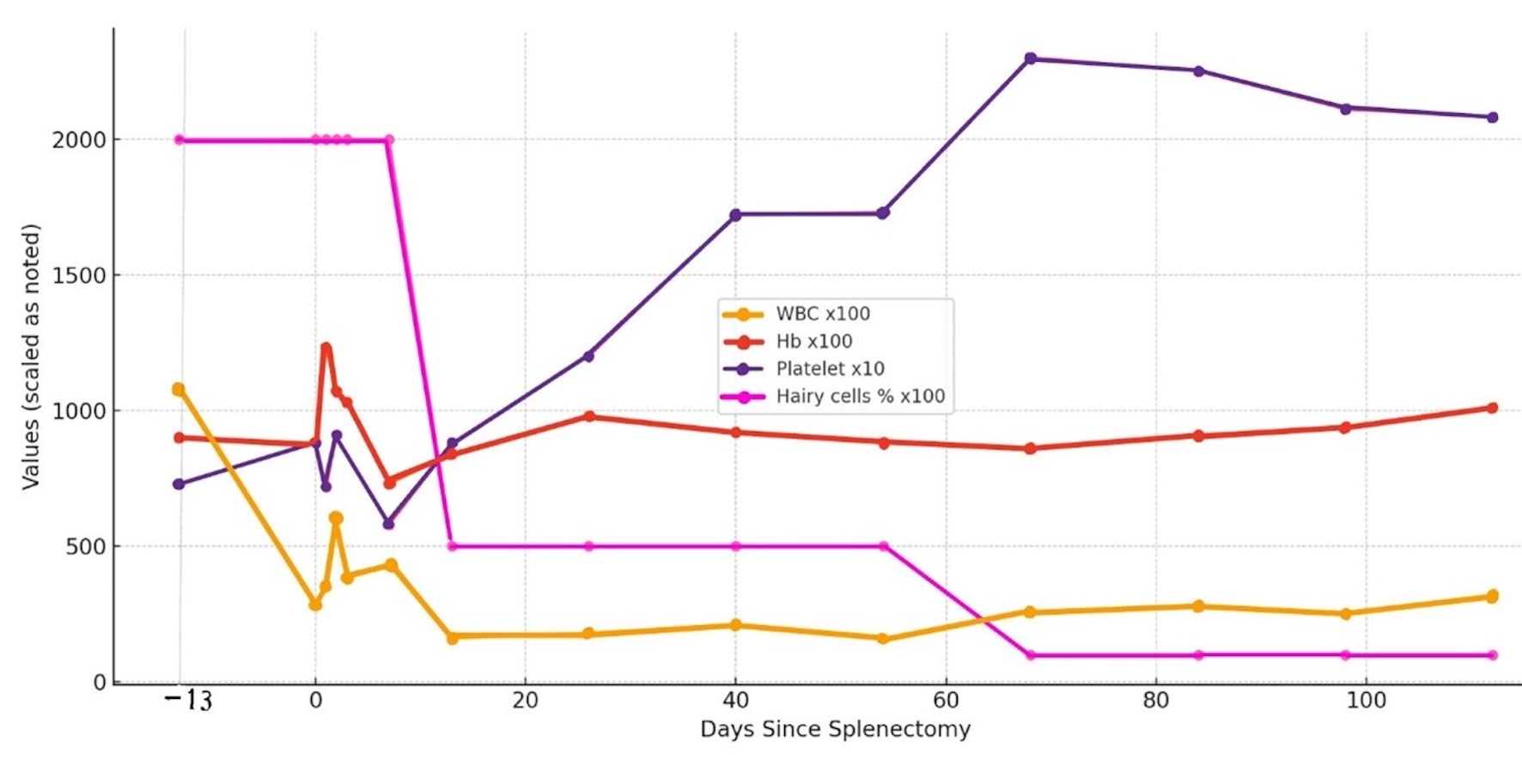 Click for large image | Figure 6. The trends of WBCs, Hb, platelet count, and circulating hairy cells are compared in an integrated graph. Hb: hemoglobin; WBCs: white blood cells. |
Hematological changes
During hospitalization, the patient developed persistent neutropenia, anemia, and thrombocytopenia until post-splenectomy day 13 when the platelet counts started to increase. The WBC counts began to improve in about 2 months and took 4 months to reach 3.2 × 103/µL post-splenectomy. Anemia with an Hb range of 8 - 9 g/dL persisted until 4 months later when the Hb level increased above 10 g/dL. The previous constitutional symptoms of easy fatiguability, weight loss, and night sweats were resolved. Circulating hairy cells significantly decreased (Table 1) and the “hairy” projections were noticeably smoother by 2 weeks post-splenectomy (Fig. 1c, d). By 2 months post-splenectomy, typical hairy cells disappeared, and mononuclear cells with slight ruffles without typical hairy projections were scarcely seen (Fig. 1e, f). Severe monocytopenia (< 0.1 × 103/µL) persisted throughout the disease course. The trends of WBC, Hb, platelet count, and circulating hairy cells were compared in an integrated graph (Fig. 6).
| Discussion | ▴Top |
Splenectomy was considered the treatment of choice for HCL until 1984 when IFN-α was first introduced. Splenectomy resulted in overall response rates of 60% to 100% and a median response duration of 5 - 20 months and with overall survival rate of 55% to 70% after 5 years [2]. It was recommended to wait at least 6 months after splenectomy before initiating further treatment approaches to reach the full benefits of splenectomy [5]. Patients with HCL benefit from splenectomy compared with non-splenectomized patients and respond better and faster to the following therapy [2]. Although splenectomy had positive impacts on pancytopenia, it failed to address the impacts of circulating hairy cells and bone marrow infiltration, ultimately allowing the disease to progress [2, 5].
The hairy projections on the surface of HCL cells are an extension of the cytoskeleton and the scanning electron microscopic examination revealed a combination of microvilli and ruffles [6]. However, the cause of the hairy projections is not known. The “hairy” morphology disappears after treatment with IFN-α [7] or BRAF inhibitors [8]. With IFN-α treatment, HCL-related surface antigen CD25 expression decreases, and the hairy projections disappear [7]. Hairy morphology appears to be dependent on BRAF V600E-mutant-mediated activation of the Ras-Raf-MEK-ERK pathway as HCL cells exposed in vitro to vemurafenib, a BRAF inhibitor, down-regulates CD25 and lost their surface hairy projections [8], which was confirmed in a clinical study [9]. However, HCL-like diseases having similar hairy projections generally do not express CD25 [4], which indicates that CD25 expression is not the sole cause of the hairy morphology. In HCL, the phosphoprotein (pp)52 is over-expressed and may play an important role in forming the hairy projections [9]. The pp52 binds to F-actin within the cell’s cytoskeleton, resulting in the formation of the hairy projections. IFN-α reduced pp52 levels, normalized intracellular pp52 distribution, and reverted hairy HCL cells to smooth B cell morphology [10].
When the bone marrow is heavily infiltrated by HCL, post-splenectomy platelet recovery is often incomplete, though partial responses can be observed due to the elimination of splenic sequestration [11]. However, in our case, despite extensive bone marrow involvement by HCL cells, thrombocytopenia was completely resolved within approximately 1 month after splenectomy (Table 1) (Fig. 6). Moreover, circulating HCL cells with characteristic hairy projections noticeably decreased post-splenectomy, correlating with thrombocytopenia recovery (Table 1) (Fig. 6). By 2 weeks post-procedure, hairy cells comprised less than 5% of WBCs, and their projections appeared smoother (Fig. 1d-f).
Elimination of splenic sequestration alone cannot fully explain the reduction of circulating HCL cells, as these cells are continuously released from the bone marrow. To our knowledge, no published reports have demonstrated an association between changes in HCL morphology and clinical improvement or disease remission.
This observation suggests that hematological recovery following splenectomy in HCL may involve mechanisms beyond the blood cell sequestration. Possible explanations for the improvement of peripheral blood cell count and the reduction in circulating HCL cells with the disappearance (or smoothing) of hairy projections after splenectomy may include: 1) Reduction in the absolute number of HCL cells: The spleen may serve as a reservoir for HCL cells. Removing the spleen reduces the overall tumor burden. 2) Morphological changes of HCL cells: The spleen may influence the hairy morphology of HCL cells. The hairy projections may become more disorganized as HCL cells pass through the narrow spaces of splenic cords and sinuses. 3) Immunological and molecular alterations: We postulate that splenectomy may induce changes in the immunological environment. The spleen plays a key role in both innate and adaptive immunity [12]. For example, unlike bone marrow-derived dendritic cells (DCs), endogenous splenic DCs have the distinct function of participating in the induction of cytotoxic T cells but not helper T cell responses [13]. Therefore, removing the spleen may modulate immune responses, potentially altering HCL cell biology. However, there is no study demonstrating molecular or immunological changes in HCL after splenectomy. 4) Potential stem cell origin in the spleen: Some patients experience prolonged remission after splenectomy [11, 14], which cannot be solely attributed to cytoreduction or elimination of a sequestration site. Myers et al hypothesized that HCL stem cells might originate in the spleen [14]. If cytoreduction of HCL cells by splenectomy is the main mechanism of the improvement, a better response is expected with a larger spleen harboring more HCL cells. The lack of correlation between spleen weight and clinical response [11] further supports this idea, suggesting splenectomy may influence HCL progression via mechanisms beyond simple removal of the spleen.
In this case, the constitutional symptoms of the patient, such as fatigue, weight loss, and night sweats, resolved following splenectomy, potentially reflecting a reduction in inflammatory cytokine production. Thrombocytopenia recovered within 2 weeks post-splenectomy, while WBC counts and Hb levels increased over 4 months. If hematological improvement were solely due to the elimination of splenic sequestration, recovery would have occurred more rapidly, and circulating HCL cells with hairy projections would not have decreased.
Current National Comprehensive Cancer Network (NCCN) guidelines (Version 1.2025) for HCL do not include splenectomy as a first-line or second-line treatment option [15]. However, splenectomy is included as a treatment option in the European Society of Medical Oncology (ESMO) guidelines in patients with certain situations: resistant massive symptomatic splenomegaly (> 10 cm below the costal margin) with accompanied “low-level bone marrow infiltration”, progressive HCL during pregnancy, disease refractory to nucleoside analogues and IFN-α [16]. ESMO restricted the indication of splenectomy to “low-level bone marrow infiltration” because splenectomy was reported not to affect the HCL infiltration but simply eliminate the splenic sequestration. However, in this case, improvement of blood cells was observed after splenectomy despite heavy bone marrow infiltration by HCL.
To our knowledge, the changes of the hairy cells’ morphology and dramatic reduction of circulating HCL cells after splenectomy have not been reported in the past. Its mechanism is not known. We postulate that splenectomy may induce molecular or immunological changes that alter HCL cell behavior, which warrants further research.
Conclusion
This case demonstrates beneficial hematological changes occurring after splenectomy in HCL, including improvement of peripheral cytopenias, a marked reduction in circulating hairy cells, and resolution of constitutional symptoms. Although splenectomy does not directly target bone marrow infiltration, its influence on HCL cell morphology, as well as its potential molecular and immunological effects, warrants further investigation.
Limitation
In this case, splenectomy was done on an urgent basis after the traumatic rupture, not as an elective surgery. The patient developed pneumonia after splenectomy and received antibiotics which could have affected his hematology recovery. During the patient’s hospitalization, he received many medications, including antibiotics. We cannot completely exclude the possibility of these medications affecting the changes observed after splenectomy.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient. The Institutional Review Committee (IRB) at Kern Medical approved this study (approval #24141).
Author Contributions
Stanley Kim provided direct patient medical care, conceived and designed the study, collected and analyzed/interpreted the data, drafted the manuscript, and revised the final version. Anna Mikami provided direct patient medical care, collected and analyzed/interpreted the data, and drafted and revised the manuscript. William Stull provided direct patient medical care, collected and analyzed/interpreted the data, drafted the manuscript, and revised the final version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Grever MR, Abdel-Wahab O, Andritsos LA, Banerji V, Barrientos J, Blachly JS, Call TG, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood. 2017;129(5):553-560.
doi pubmed - Maevis V, Mey U, Schmidt-Wolf G, Schmidt-Wolf IG. Hairy cell leukemia: short review, today's recommendations and outlook. Blood Cancer J. 2014;4(2):e184.
doi pubmed - Bagrodia N, Button AM, Spanheimer PM, Belding-Schmitt ME, Rosenstein LJ, Mezhir JJ. Morbidity and mortality following elective splenectomy for benign and malignant hematologic conditions: analysis of the American College of Surgeons National Surgical Quality Improvement Program data. JAMA Surg. 2014;149(10):1022-1029.
doi pubmed - Troussard X, Maitre E. Untangling hairy cell leukaemia (HCL) variant and other HCL-like disorders: Diagnosis and treatment. J Cell Mol Med. 2024;28(3):e18060.
doi pubmed - Jones G, Parry-Jones N, Wilkins B, Else M, Catovsky D, British Committee for Standards in H. Revised guidelines for the diagnosis and management of hairy cell leukaemia and hairy cell leukaemia variant*. Br J Haematol. 2012;156(2):186-195.
doi pubmed - Gamliel H, Golomb HM. Unique scanning electron microscopic features of hairy cells in hairy-cell leukemia. A review and current status. Scan Electron Microsc. 1986;(Pt 4):1515-1521.
pubmed - Mantovani G, Astara G, Curreli L, Lai P, Turnu E, Locci F, Lantini MS, et al. Ultrastructural, immunologic and clinical follow-up of five patients with HCL treated with interferon (IFN) for more than three years. Haematologica. 1992;77(4):326-335.
pubmed - Pettirossi V, Santi A, Imperi E, Russo G, Pucciarini A, Bigerna B, Schiavoni G, et al. BRAF inhibitors reverse the unique molecular signature and phenotype of hairy cell leukemia and exert potent antileukemic activity. Blood. 2015;125(8):1207-1216.
doi pubmed - Tiacci E, Park JH, De Carolis L, Chung SS, Broccoli A, Scott S, Zaja F, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373(18):1733-1747.
doi pubmed - Miyoshi EK, Stewart PL, Kincade PW, Lee MB, Thompson AA, Wall R. Aberrant expression and localization of the cytoskeleton-binding pp52 (LSP1) protein in hairy cell leukemia. Leuk Res. 2001;25(1):57-67.
doi pubmed - Golomb HM, Vardiman JW. Response to splenectomy in 65 patients with hairy cell leukemia: an evaluation of spleen weight and bone marrow involvement. Blood. 1983;61(2):349-352.
pubmed - Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2023;56(5):1152.
doi pubmed - Hey YY, O'Neill HC. Murine spleen contains a diversity of myeloid and dendritic cells distinct in antigen presenting function. J Cell Mol Med. 2012;16(11):2611-2619.
doi pubmed - Myers TJ, Ikeda Y, Schwartz S, Pharmakidis AB, Baldini MG. Primary splenic hairy cell leukemia—remission for 21 years following splenectomy. Am J Hematol. 1981;11(3):299-303.
doi pubmed - https://www.nccn.org/professionals/physician_gls/pdf/hairy_cell.pdf.
- Robak T, Matutes E, Catovsky D, Zinzani PL, Buske C, Committee EG. Hairy cell leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v100-107.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.