| Journal of Hematology, ISSN 1927-1212 print, 1927-1220 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Hematol and Elmer Press Inc |
| Journal website https://jh.elmerpub.com |
Case Report
Volume 13, Number 6, December 2024, pages 295-299
A Case of Fulminant Cerebral Edema Leading to Death After Chimeric Antigen Receptor T-Cell Therapy
Katherine Hickmanna, Rachel DiLeob, Kathleen Faringerc, Chelsea Petersonc, Cyrus Khanc, Yazan Samhouric, d
aDrexel University College of Medicine, Philadelphia, PA, USA
bDepartment of Internal Medicine, Allegheny Health Network, Pittsburgh, PA, USA
cDivision of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA
dCorresponding Author: Yazan Samhouri, Division of Hematology and Cellular Therapy, Allegheny Health Network Cancer Institute, Pittsburgh, PA 15224, USA
Manuscript submitted October 10, 2024, accepted November 14, 2024, published online December 2, 2024
Short title: Fatal Cerebral Edema in CAR T
doi: https://doi.org/10.14740/jh1367
| Abstract | ▴Top |
Chimeric antigen receptor (CAR) T-cell therapy has transformed treatment of refractory B-cell malignancies; however, treatment puts patients at risk for side effects secondary to the amplified immune response it induces. Fulminant cerebral edema (FCE) is one of the rarest, yet most devastating side effects following CAR T-cell therapy. Due to this rarity, FCE has not been well characterized and the risk factors associated with its development are not fully understood. Here, we present a case of a 42-year-old male who passed away from FCE following CAR T-cell infusion with the primary goal to better understand which patients are at higher risk of developing FCE before and after infusion.
Keywords: CAR T-cell; Fulminant cerebral edema; CRS; ICANS
| Introduction | ▴Top |
Chimeric antigen receptor (CAR) T-cell therapy has changed the treatment scheme of B-cell lymphoid malignancies and expanded into other applications since its approval in 2017. However, treatment still poses significant risk of side effects given the inflammatory response CAR T-cell proliferation induces. The most common side effect experienced is systemic inflammation also known as cytokine release syndrome (CRS) [1]. Another frequent occurrence is immune effector cell-associated neurotoxicity syndrome (ICANS), which is the development of neurological symptoms that has been reported in other immunotherapy treatments [2]. Both CRS and ICANS are a spectrum of disease with corresponding severity scales. ICANS is measured most often using the immune effector cell encephalopathy (ICE) score, with a lower score indicating worse status.
CRS pathophysiology is better understood, with the known involvement of interleukin (IL)-6, leading to the development of tocilizumab, an IL-6 receptor inhibitor, approved for the treatment of CRS [3]. CRS often precedes ICANS, which suggests a connection in underlying pathophysiology, but not all cases of ICANS have been responsive to classic treatment of CRS [4].
Here we present a case of a 42-year-old male who passed away from fulminant cerebral edema (FCE) following CAR T-cell infusion in hopes to better understand which patients are at increased risk before and after infusion.
| Case Report | ▴Top |
The patient is a 42-year-old male diagnosed with stage III mantle cell lymphoma in 2017 (TP53 mutant). He received five cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP). Due to disease progression on R-CHOP, he then started ibrutinib in 2018. He achieved complete response with ibrutinib. However, it was discontinued in 2022 when he developed a rash secondary to ibrutinib. Five months later, he had disease recurrence and was restarted on ibrutinib. He again achieved complete response until symptom recurrence in 2023.
At that time, since he was refractory to multiple therapies, a decision was made to pursue CAR T-cell therapy using Brexucabtagene autoleucel (Brexu-cel). Prior to CAR T-cell therapy, he started pirtobrutinib as bridging therapy. The evening after the infusion, the patient experienced an episode of tachycardia to the 100 - 120 bpm range and hypotension of 89/69 (baseline of 90s/60s), which responded to IV hydration. Tachycardia returned on day +2 in addition to a fever of 102 °F. With these symptoms, he met the criteria for CRS grade 1 and received the first dose of tocilizumab. At this time, he was not experiencing any neurological symptoms, grade 0 ICANS.
However, overnight on day +4, he had grade 3 CRS with hypotension and hypoxia, so a second dose of tocilizumab was given. Later on, he developed lethargy and was minimally responsive with subsequent worsening respiratory status with stridor. Due to concern for ability to protect his airway, he was sedated and intubated. Intubation was successful after one attempt and there were no signs of laryngeal edema as a cause of stridor. He was started on pulse dose steroids 500 mg of IV methyl prednisone every 12 h for presumed grade 4 CRS. ICE score for ICANS was unable to be completed due to acute altered mental status (AMS), thus making him ICANS grade 4. Due to unclear cause of such acute mental status change, an electroencephalogram (EEG) was ordered, and neurology was consulted to evaluate for seizures. A CT of the head (CTH) was ordered at the time which was unremarkable for any edema (Fig. 1). The patient was started on prophylactic dose of levetiracetam 750 mg twice a day preceding these events. EEG did not show active epileptic activity but did show low voltage on the right side, concerning for either toxic metabolic etiologies or the left-right disparity suggesting a structural lesion, with magnetic resonance imaging (MRI) recommended. An MRI was ordered, but unable to be completed due to subsequent rapid deterioration. There was low concern at this time for infectious etiology of AMS due to speed of onset and patient on multiple days of broad-spectrum antibiotics for neutropenic fever and neutropenic bacterial, viral and fungal infection prophylaxis. The patient’s ferritin and C-reactive protein (CRP) levels were checked throughout their course and are represented in Figure 2.
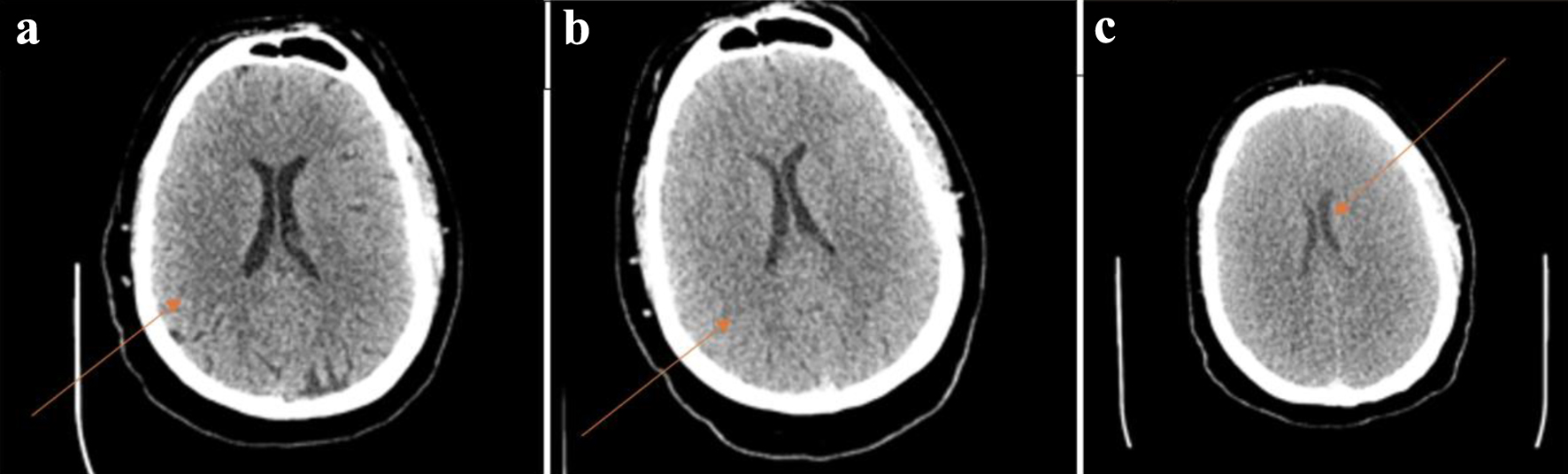 Click for large image | Figure 1. (a) Normal head CT performed at 13:08 on day +4 with arrow indicating intact gray-white matter differentiation. (b) Head CT showing diffuse cerebral edema at 17:18 on day +4 with arrow indicating comparative loss of gray-white matter differentiation. (c) CT showing worsening of cerebral edema at 21:50 on day +4 with arrow demonstrating ventricle compression due to severe edema. CT: computed tomography. |
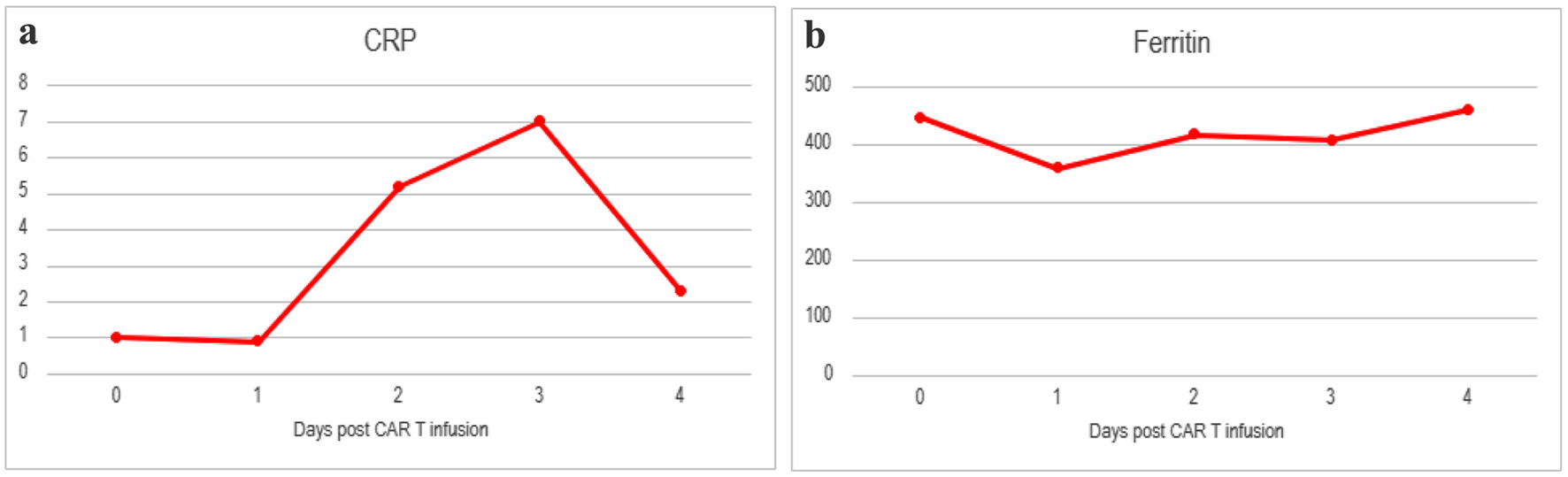 Click for large image | Figure 2. Inflammatory markers including (a) CRP and (b) ferritin for patient from day of CAR T-cell infusion until death. Baseline CRP 1.0 and ferritin 186. CAR: chimeric antigen receptor; CRP: C-reactive protein. |
He continued to not respond to stimuli, but cough, gag, and corneal reflexes were intact. To rule out thrombosis, a computed tomography angiography (CTA) was performed and showed no large vessel occlusive finding, but concurrent CTH 4 h after the first one showed diffuse cerebral edema (Fig. 1). He was given hypertonic saline and mannitol in addition to steroids to help manage the edema. At 7:30 pm, he experienced seizure like activity and was given lorazepam and started on a propofol drip, in addition to levetiracetam loading dose. Hyperventilation was started and ventriculostomy was considered but determined unlikely to help. His symptoms did not improve with the cerebral edema and seizure treatments. The patient’s neurological exam progressed, and his pupils became fixed and unresponsive. Due to concern for current or impending herniation, a repeat CTH 9 h after the initial scan showed worsening cerebral edema (Fig. 1). On a repeat EEG, it confirmed lack of electrical activity. On early morning of day +5, he was pronounced brain dead. His family made the decision to extubate, and he passed shortly after.
| Discussion | ▴Top |
FCE has been described in the literature in the case of a 35-year-old woman who passed away within 24 h after CAR T-cell infusion [1]. FCE is characterized by rapid onset cerebral edema that often does not respond to intravenous steroids and leads to brain death. The underlying pathophysiology in the setting of CAR T-cell therapy has not been well characterized and there is limited guidance on associated risk factors, early recognition, and treatment. One proposition regarding CRS and ICANS severity is that younger patients with a more robust immune system are more at risk due to increased cytokine response [5]. Our patient’s inflammatory markers throughout the hospital course can be found in Figure 2. Other cases of FCE following CAR T-cell are summarized in Table 1 [1, 4, 6].
 Click to view | Table 1. Cases of FCE and Their Characteristics |
Due to the correlation between severity of CRS and ICANS with CRS often preceding ICANS, it has been hypothesized that ICANS is a result of systemic inflammation. Specifically, it has been theorized that systemic inflammation disrupts the blood-brain barrier (BBB) [7]. Based on various inflammatory markers, this disruption has been posited to occur at the endothelial, astrocyte or microglial cell [2]. However, although rare, it should be noted that ICANS can develop independently of CRS making the underlying pathophysiology even more elusive [8]. It is important to note that ICANS typically results in minimal longstanding neurological deficits; however, in the case of FCE, it is most often fatal and unresponsive to typical treatment, which may demonstrate that it is a separate entity from ICANS [2].
In the case of fatal FCE described in Torre et al [4], there was an autopsy performed to better elucidate the underlying pathology. Findings included a brain with markedly increased weight, flattened gyri, cerebral aqueduct compression, but surprisingly no brain herniation. On the histological assessment, there were signs of endothelial, BBB, and astrocyte damage. There were no signs of cancer cells, CAR T-cells, or infection. Although this does not provide a definitive answer, it does provide pertinent negatives.
Some cytokines have been connected to one, or both CRS and ICANS. IL-6 is one known driver of CRS hence treatment with tocilizumab, an IL-6 receptor inhibitor. At one point it was postulated that there may be a connection between treatment with tocilizumab and increased risk of ICANS [3]. This is due to the inhibition not being directed toward IL-6 itself but acting on receptors. This possibly leads to higher IL-6 concentrations in the blood for penetration into the central nervous system (CNS). There have been proposals to transition to a direct IL-6 inhibitor like siltuximab, but new data from Gust et al [3] show that the original hypothesis may be false. In terms of ICANS itself, the cytokines most associated are IL-1, IL-15, granulocyte-macrophage colony-stimulating factor (GMCSF), and interferon (INF)-gamma. IL-10 also has an association, but this is most likely due to its inhibitory action in the setting of a severe inflammatory state [2].
One key piece in determining risk factors for the developments of FCE is the findings of the ROCKET trial that was discontinued early due to multiple cases of FCE [5]. The risk factors that were identified were age < 30 years, treatment less than or equal to two regimens, intensive bridging chemotherapy, high-intensity lymphodepletion with fludarabine and cyclophosphamide. Our patient had the associated risk factors of high-intensity lymphodepletion and two prior regimens. Additionally, although not less than 30 years of age, he is still young in comparison to the median age of CAR T treatment of 65 [9]. Some risk factors that have been proposed but are disproven or inconclusive at this point include baseline neurological disease, type of CAR T-cell product used, or CNS malignancy involvement.
Given the cytokine profile associated with ICANS, various treatments have been both proposed and utilized. For IL-1 blockade there is anakinra, currently used for rheumatological disorders, which has current ongoing clinical trials for treatment of ICANS [10]. For IL-15 blockade, there is not currently a used antibody, and it has not been proposed in trials for use in this setting due to the likelihood of negative effect on tumor response to CAR T-cell therapy. However, given the strong association in review by Gust et al [2], with the development of severe ICANS, it may be a consideration for cases like the one presented here where preservation of life might outweigh the possibility of incomplete response. Intrathecal chemotherapy is another option in refractory cases [11].
Based on our case and the literature review of cases in Table 1, information provided by the pathology in the Torre case and review done by Gust et al comparing the cytokine involvements in ICANS versus severe ICANS, a mechanism arises. The IL-1B release from CAR T-cell induced inflammation causes initial BBB dysfunction and release of IFN-gamma by microglial cells. IFN-gamma was shown to cause astrocyte swelling, which can induce electrolyte disturbances resulting in neuron dysfunction and swelling. This swelling disrupts contact of the astrocyte podocytes which worsens BBB dysfunction allowing for this process to repeat itself indefinitely leading to the picture we see in FCE. If this is the case, early initiation of treatment options like anakinra would be of the utmost importance. One challenge in prompt treatment may be the delay in imaging findings. As in our case, the patient was most likely on their way to developing cerebral edema at the time of intubation, but negative imaging provided false reassurance. This may point to a need to initiate treatment based more on clinical neurological picture than imaging until we have a better system to predict patients at risk for impending FCE accurately.
Conclusion
We describe this case with the hope that with more data in the literature on cases like our patient, in the future we will be able to recognize the symptoms, treat them earlier, more effectively, and even predict those at risk. As others have mentioned, in the future judicious monitoring and reporting of these outcomes, it is of the utmost importance to better understand the true incidence of FCE and other rare events with CAR T-cell therapy.
Acknowledgments
None to declare.
Financial Disclosure
This case report was not funded.
Conflict of Interest
All authors have no conflict of interest.
Informed Consent
Informed consent has been obtained for treatment of CAR T-cell therapy, which was approved for Mantel Cell Lymphoma prior to treatment date.
Author Contributions
Yazan Samhouri MD had full access to all the data and analysis in the study and takes responsibility for the integrity of data and the accuracy of the data analysis. Concept and design: Yazan Samhouri. Drafting of the manuscript: all authors. Critical revision of the manuscript: all authors. Administrative and technical support: Yazan Samhouri. Supervision: Yazan Samhouri.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BBB: blood-brain barrier; Brexu-cel: brexucabtagene autoleucel; CAR: chimeric antigen receptor; CRS: cytokine release syndrome; CTH: computed tomography of the head; EEG: electroencephalogram; FCE: fulminant cerebral edema; ICANS: immune effector cell-associated neurotoxicity syndrome; ICE: immune effector cell encephalopathy; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone
| References | ▴Top |
- Pensato U, Muccioli L, Zinzani P, D'Angelo R, Pierucci E, Casadei B, Dicataldo M, et al. Fulminant cerebral edema following CAR T-cell therapy: case report and pathophysiological insights from literature review. J Neurol. 2022;269(8):4560-4563.
doi pubmed - Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. 2020;11:577027.
doi pubmed - Gust J, Finney OC, Li D, Brakke HM, Hicks RM, Futrell RB, Gamble DN, et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86(1):42-54.
doi pubmed - Torre M, Solomon IH, Sutherland CL, Nikiforow S, DeAngelo DJ, Stone RM, Vaitkevicius H, et al. Neuropathology of a case with fatal CAR T-cell-associated cerebral edema. J Neuropathol Exp Neurol. 2018;77(10):877-882.
doi pubmed - DeAngelo DJGA, Park JH, et al. Clinical outcomes for the phase 2, single-arm, multicenter trial of JCAR015 in adult B-ALL (ROCKET Study). Society for Immunotherapy in Cancer (SITC). 2017.
- Santomasso BD, Gust J, Perna F. How I treat unique and difficult-to-manage cases of CAR T-cell therapy-associated neurotoxicity. Blood. 2023;141(20):2443-2451.
doi pubmed - Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419.
doi pubmed - Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32(12):1091-1101.
doi pubmed - Berning P, Shumilov E, Maulhardt M, Boyadzhiev H, Kerkhoff A, Call S, Reicherts C, et al. Chimeric antigen receptor-T cell therapy shows similar efficacy and toxicity in patients with diffuse large B-cell lymphoma aged 70 and older compared to younger patients: A multicenter cohort study. Hemasphere. 2024;8(3):e54.
doi pubmed - Park JH, Nath K, Devlin SM, Sauter CS, Palomba ML, Shah G, Dahi P, et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med. 2023;29(7):1710-1717.
doi pubmed - Asawa P, Vusqa U, Khan C, Samhouri Y, Fazal S. Intrathecal chemotherapy as a potential treatment for steroid-refractory immune effector cell-associated neurotoxicity syndrome. Anticancer Res. 2022;42(8):3853-3856.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Hematology is published by Elmer Press Inc.